Tripsacum dactyloides (L.) L. var. occidentale Cutler & Anders.
Scientific Name: Tripsacum dactyloides (L.) L. var. occidentale Cutler & Anders.

| General Information | |
|---|---|
| Usda Symbol | TRDAO |
| Group | Monocot |
| Life Cycle | Perennial |
| Growth Habits | Graminoid |
| Native Locations | TRDAO |
Plant Guide
Alternate Names
Tripsacum dactyloides (L.) L. var. occidentale Cutler & Anders., Coix dactyloides L., bullgrass, capim gigante, eastern mock gama, fakahatchee grass, Gamagras, herbe grama, maicillo oriental, pasto Guatemala, wild corn, zacate maicero
Uses
Forage: The major use of eastern gamagrass is as a forage crop. It is highly productive as intensively managed pasture, hay and silage. Eastern gamagrass can be developed into an important component of forage for beef and dairy production systems (Dewald et al., 2006). Since eastern gamagrass is a warm-season grass, the distribution of yield throughout the summer makes it a useful source of forage when cool-season grasses such as tall fescue (Schedonorus phoenix) are relatively unproductive or dormant (Roberts & Kallenbach, 2006). Forage quality: Researchers have evaluated eastern gamagrass forage quality across many locations, soil fertility levels and genotypes (Coblentz et al., 1999; Douglas et al., 2000a; Edwards et al., 2000; Salon et al., 2000). The crude protein percentage measurements on gamagrass forage at the boot growth stage average about 12.5. The boot stage is when the inflorescence is enclosed in the sheath of the uppermost leaf. The in vitro digestibility percentage measurements on gamagrass forage average about 70. Forage yield and animal performance: The average daily gains of steers grazing continuously on eastern gamagrass across several studies conducted in the middle south ranged between 1.1 to 2.2 lb per day (Aiken, 1997; Burns et al., 1992). In North Carolina steers continuously grazing either ‘Pete’ eastern gamagrass, ‘Carostan’ flaccidgrass (Pennisetum flaccidum) or ‘Coastal’ bermudagrass (Cynodon dactylon) exhibited average daily gains of 1.8, 1.5 and 0.7 lb, respectively (Burns & Fisher, 2000). A series of studies conducted in North Carolina show that across the total grazing season the average daily gain of steers grazing either Pete eastern gamagrass or ‘Kanlow’ switchgrass (Panicum virgatum) was about 2 lb. In contrast, the average daily gain for steers grazing the widely used tall fescue-bermudagrass system was about 1.6 lb during the same period. During a dry year in North Carolina (rainfall during the pasture season about 10 inches below the average), ‘Alamo’ switchgrass was the most productive forage as defined by steer gain per acre; but ‘Iuka IV’ eastern gamagrass was more productive than ‘Rountree’ big bluestem (Andropogon gerardii), Caucasian bluestem, and bermudagrass. In western Kentucky, cow-calf pairs were grazed on either eastern gamagrass or Caucasian bluestem (Bothriochloa bladhii) and bermudagrass during June though early September (Pingel, 1999). The data from this study was not statistically analyzed. But, the author observed that eastern gamagrass maintained more consistent forage production across the grazing season than either Caucasian bluestem or bermudagrass. Gamagrass forage production did not decrease as rapidly as that of Caucasian bluestem and bermudagrass across an August-September low rainfall period. Gamagrass provided fewer grazing days than either Caucasian bluestem or bermudagrass because of gamagrass’ requirement for a long rest period between grazing cycles. Filter strip, Vegetative barrier, Nutrient management: In studies conducted in Arkansas and Maryland, the harvest of eastern gamagrass aboveground tissue removed more nitrogen from soil that was fertilized with high levels of poultry litter than the harvest of yellow bluestem (Bothriochloa ischaemum var. ischaemum), but less than the harvest of switchgrass (Staver, 2000; Tharel, 2000). Only about 12% of the phosphorous applied in the poultry litter was accounted for in aboveground tissue. Therefore, using poultry litter to supply the nitrogen requirements of either gamagrass or switchgrass would result in a rapid increase in soil phosphorous levels. McLaughlin et al. (2004), in a study conducted in east-central Mississippi, compared 6 grasses: common and Coastal bermudagrass, Pete eastern gamagrass, ‘Lometa’ Indiangrass (Sorghastrum nutans), Johnsongrass (Sorghum halepense) and Alamo switchgrass for the uptake of both macro and micronutrients. Swine effluent spray was applied to the soil for 8 years prior to the initiation of the experiment and during the first 2 years of the 3 year study. Bermudagrass, both Coastal and common, accumulated more nitrogen and phosphorous per acre into above-ground herbage than the other grasses (P<0.05). The N/P uptake ratios ranged from 4.7 for Costal bermudagrass to 9.3 for eastern gamagrass. When the objective of a swine waste nutrient management hay system is to provide nitrogen for optimum forage production while avoiding accumulation of excess phosphorous in the soil, forages with N/P ratios less than 10 are expected to satisfy the objective, but forages with ratios greater than 10 are not. The N/P uptake ratios of all 6 grasses were less than 10.0, with common bermudagrass and Indiangrass displaying the lowest ratios, and eastern gamagrass the highest. In summary, the performance of the bermudagrass exceeded those of the other 4 grasses 83% of the time for dry matter yield and 76% of the time for nutrient uptake. The authors conclude “among the 6 grasses tested, common bermudagrass is the best choice for replacing Johnsongrass as a warm-season perennial grass hay crop for nutrient management in this swine effluent spray field.” But, the native species, eastern gamagrass and switchgrass could be used in the current nutrient management system. In Mississippi, eastern gamagrass, giant reed (Arundo donax), big bluestem, Alamo switchgrass, and tall fescue established in filter strips adjacent to cotton fields were equally effective in reducing sediment runoff. Consequently, the grasses were equally effective in trapping soil applied herbicides that were either attached or adsorbed to the soil (Rankins, 1998). Eastern gamagrass, when compared to tall fescue, increased the infiltration of water and improved soil physical and hydraulic properties (Perrygo et al. 2001). The authors recommend planting eastern gamagrass as filter strips along the edges of agricultural fields to enhance infiltration and reduce surface runoff. Eastern gamagrass has received considerable attention for use in vegetative barriers for soil erosion control because the crown has the capacity to elevate coarse aerial foliage above sediment deposition and to anchor the plant with stout brace roots. Researchers in northern Mississippi compared vegetative barriers composed of 1, 2, 3 or 4 rows of either eastern gamagrass or switchgrass (Becker, 2001; Dewald et al., 1996). One and two-row barriers were as resistant as three and four-row barriers to overtopping by flowing water that contained sediment. Switchgrass remained more erect and held back more water and sediment than eastern gamagrass.
Status
Please consult the PLANTS Web site and your State Department of Natural Resources for this plant’s current status (e.g. threatened or endangered species, state noxious status, and wetland indicator values).
Description
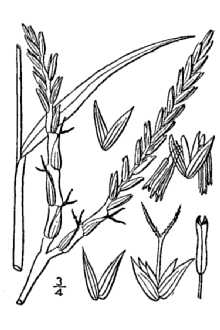
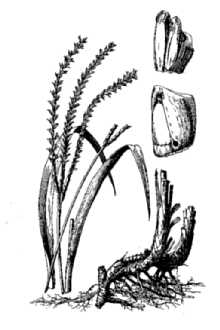
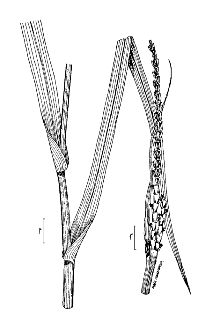
General: Grass Family (Poaceae), tribe Andropogoneae, and subtribe Tripsacinae. It shares the same subtribe as corn (Zea mays). Eastern gamagrass is a native, perennial, bunchgrass and is a distant relative of corn. It is a long-lived (to 50 years), warm-season species native to most of the eastern half of the United States. It ranges in height from 4 to 8 feet. The leaf blades are flat, long (12 to 30 inches) and wide (0.4 to 1.2 inches), with a well-defined midrib. It reproduces vegetatively from thick, knotty rhizome like structures called proaxes. The inflorescences have 1 to 3 racemes. The spikes are 6 to 10 inches long. Similar to corn, eastern gamagrass has separate male and female flowers (monoecious). But unlike corn, each gamagrass spike contains both male and female flowers. Male flowers occupy the top ¾ of the spike and female flowers the bottom ¼. Terminal inflorescences occur on the top of the stem, and lateral inflorescences occur at the leaf axil, which is the angle between the leaf and stem. Seed is produced from June to September resulting in uneven maturation. The spike ripens from the top down and is susceptible to shattering. The seed yield of gamagrass seed is low, and it does not reseed adequately from established stands. Eastern gamagrass occurs predominately at the diploid (2n = 2x = 36) and tetraploid (2n = 4x = 72) levels although triploids (2n = 3x = 54), pentaploids (2n = 5x = 90), and hexaploids (2n = 6x = 108) have been reported (Farquharson, 1995). Only the diploid plants are sexual and cross-pollinated. The tetraploids and the rest of the polyploids are apomictic (Burson et al., 1990). They produce seed asexually that is genetically identical to the mother plant. Distribution: For current distribution, please consult the Plant Profile page for this species on the PLANTS Web site.
Adaptation
The indigenous U.S. range of eastern gamagrass extends from central Texas to southeastern Nebraska and central Iowa and east to the Atlantic Ocean (Rechenthin, 1951). Eastern gamagrass’ range extends to Central and South America and the Caribbean (Tropical Forages, 2006). Gamagrass flourishes under dryland conditions where annual precipitation exceeds 35 inches (Dewald et al., 2006). Respectable forage production can be achieved on good soils in regions with 25 inches of annual precipitation. It can be grown in areas with less annual precipitation on irrigated and sub-irrigated land. The eastern gamagrass cultivars Pete and Iuka IV exhibited yellow leaves when grown at Los Lunas, New Mexico on a Belen loam (J. Henson, personal communication, 2001). A pH range of 7.9 to 9.0 characterizes the Belen loam. Presumably, the yellow leaves are the result of iron deficiency due to the inability of gamagrass to take-up iron from the high pH soil. A suggested minimum soil pH for eastern gamagrass is 5.1 (Foy et al.1999) and the maximum is 7.5 (Sharp Brothers Seed Company, 1999). Another estimate of the optimum pH range is 5.4 to 6.0 (Roberts & Kallenbach, 2006). Gamagrass is intolerant of saline soils. Eastern gamagrass culm height was reduced when grown in a soil with an electrical conductivity of 0.72 dS/m in New Mexico (Henson, 1993). In Kansas, Iuka IV was established on a commercial seed production field, with a soil pH range from 6.9 to 8.0. A good stand exists across the whole field, but both seed and forage production were less in the area of the field where the soil pH is 8.0 than where the soil pH is less than 8.0. In the Northeast, gamagrass is susceptible to frost heaving on poorly drained soils. In some years this may also occur on sites with moderately well drained soils during the spring following establishment. Clay pan soils and other soils with high mechanical impedance, low pH, and frequent waterlogged conditions cover extensive areas of the Midwest, Northeast and Southeast. Clay pans are usually hard when dry, and plastic and sticky when wet. Clay pan layers hinder root growth into the soil, are acidic (pH <5.0), and may contain toxic levels of aluminum. In a study conducted in Missouri, roots of native eastern gamagrass stands effectively penetrated clay pan layers with clay contents of 30-50% clay (Clark et al., 1998). Eastern gamagrass formed extensive root channels in the clay pan layer especially where the gamagrass had been growing for more than 50 years. In a series of experiments conducted in greenhouses, eastern gamagrass exhibited tolerance to low soil pH, high soil aluminum concentrations and high soil strength (Foy, 1997; Gilker et al., 2002).Root growth of eastern gamagrass, in contrast to Sudangrass (Sorghum bicolor ssp. drummondii) was not inhibited by either acid or aluminum-toxic soil conditions. Neither low pH nor high soil strength adversely affected gamagrass root growth. The authors conclude, “The characteristics of tolerance to acid and aluminum and to high soil strength conditions make eastern gamagrass valuable in establishing grassed buffers, vegetative conservation barriers, and pastures.” Foy et al. (1999) determined the effect of liming a compact, acid, high aluminum soil in Maryland on the forage yield of Pete eastern gamagrass. The limed and non-limed soils had a pH of 5.8 and 5.1, respectively. The gamagrass annual forage yield averaged 3.25 tons/acre across two years. There was no difference between the limed and non-limed soil for forage yield. The high tolerance of eastern gamagrass to low soil pH and aluminum toxicity is in contrast to most crop plants. Krizek et al. (2003) conducted a 4 year (1997-2000) study in Maryland to determine the forage yield of Pete eastern gamagrass, when grown on an acid, compact soil. Gamagrass annual forage yield remained relatively high, averaging 2 tons/acre, during 1997 to 2000 despite moisture deficits during each of these years, and a severe moisture deficit during 1999. In contrast, adjacent plots of corn and soybean (Glycine max) exhibited severe stunting and reduced or zero grain yields during this period. Observations of gamagrass roots from pits dug adjacent to the plots indicate that gamagrass roots can penetrate acid, compact clay pans to a depth of 3 to 6.5 feet. The authors state, “The fact that eastern gamagrass can withstand periods of moisture deficits may be related to its ability to send its roots deep into the soil early in the spring when the water table is frequently perched (as at our site), thereby enabling it to tap this reservoir of water when surface moisture is limiting. . . When properly fertilized, eastern gamagrass is ideally suited for reclamation of acid and compact soils and for production of high biomass of high quality forage.”
Establishment
Since eastern gamagrass is very palatable to livestock it is one of the first grasses that is eliminated from mixed stands by grazing. Due to its high palatability in grazing systems, it is best to establish and utilize gamagrass in pure stands. However, John Dickerson, NRCS plant materials specialist, states, “Other species, particularly other native grasses, are compatible with eastern gamagrass in forage production fields if grazing and hay management is focused on the gamagrass. Also, a mixture of grasses may exclude weeds more effectively than a solid stand of gamagrass.” Seed dormancy: The caryopsis of eastern gamagrass is surrounded by a hard fruit-case. The botanical name for this fruitcase is cupule. Henceforth an eastern gamagrass caryopsis and the surrounding cupulate fruitcase is referred to as a seed unit (Galinat, 1956). Eastern gamagrass establishment is hindered by seed unit dormancy (Ahring & Frank, 1968). A cold moist stratification, which softens the cupule is the most practical method to reduce the percentage of dormant seed units (Anderson, 1985). Winter planting of dormant seed units: One alternative is to plant non-stratified seed units during autumn or winter when the soil temperature in below 50 °F, and rely on the cold, moist conditions of winter to stratify the seed (Graves et al., 1997; Gamagrass Growers Guide, 2005). If the seed units are planted too early in autumn, germination may occur during winter, which will increase the potential for winter injury. The recommended autumn/winter planting windows for several regions are available (USDA-NRCS-NPDC, 2007). Spring planting of artificially stratified seed units: An artificial stratification of seed units in a cold cabinet will break gamagrass seed dormancy. The seed units must be stratified (exposed to cold, wet conditions) for 3 to 10 weeks prior to spring sowing (Row, 1998; Springer et al., 2001). A frequently used technique for artificial stratification (Graves et al. 1997; J. Grabowski, personal communication, 2005) is the following: Fill a burlap bag about ½ full with eastern gamagrass seed units. Soak the bag containing the seed units in a 1% solution of a fungicide such as Thiram1™ for 10 to 12 hours. Check the label for clearance before using any fungicide. Drain the burlap bag that contains the seed units. Seal the burlap bag and then seal the burlap bag in a plastic bag. Store the treated seed units for 6 to 10 weeks at 35-45 °F. Do not freeze. When the seed units are removed from the cold cabinet, they should not be allowed to dry before planting. The soil must remain moist after planting artificially stratified seed until the seed germinates. Graves et al. (1997) recommends planting into a moist seed bed because if the seed dries out before germination the stand will be dramatically reduced. Some commercial seed producers sell pre-stratified seed units. These seed units are hydrated when purchased. A farmer must either plant pre-stratified seed units within 24 hours after receiving them or store the seed units in a moist-cold environment, otherwise the seed units will sprout prior to planting. A commercial seed company, Gamagrass Seed Company (Falls City, NE) has developed a proprietary process (Germtec II1 TM), which is a priming process for eastern gamagrass seed units. The advantage of primed seed over pre-stratified seed units is that primed seed units are stable during shipping without moist-cold storage and immediate planting is not required. However, the germination percentage of primed seed units declines slowly across time (Krizek et al., 2000). The recommended date for spring planting of eastern gamagrass stratified seed units is similar to that for corn (Graves et al., 1997). Roberts and Kallenbackn (2006) recommend planting stratified seed units in Missouri when soil temperatures reach 65 °F. Janet Grabowski recommends planting stratified ‘Highlander’ eastern gamagrass in Mississippi when soil temperatures are about 85 °F (J. Grabowski, personal communication, 2005). Planting techniques: Good stands of the cultivars Pete and Iuka IV eastern gamagrass have been obtained using 8 to 10 lb of high quality seed units per acre [about 45,000 to 56,250 pure live seeds (PLS) per acre] (Dewald et al., 2006). Good quality seed of the cultivars Pete and Iuka IV, which are composed of Great Plains ecotypes, usually have 5000 to 7000 seed units per lb. Higher seeding densities (12-15 lb seed units/acre) of these cultivars should be used if the number of seed units per pound is less than 6000. In general, tetraploid ecotypes with Southeast origins have larger seed units and fewer seed units per pound than ecotypes from the Great Plains. The weight per seed unit of the cultivar ‘Jackson’, which is a tetraploid ecotype of southeast Texas origin, is larger than that of both Pete and Iuka IV. Therefore, Jackson should be planted at a minimum of 10 lb of seed units per acre (J. Alderson, personal communication, 2001). The cultivar Highlander, a tetraploid ecotype with a northeast Tennessee origin, has about 2800 seed units per lb of high quality seed (J. Douglas, personal communication, 2001). Suggested seeding densities for Highlander are; 13 to 27 lb seed units per acre with a minimum row width of 24 inches when the planting will be used for forage, and 9 to 19 lb seed units per acre with a minimum row width of 36 inches when the planting will be used for seed production (J. Grabowski, personal communication, 2005). Row planters (corn, cotton, peanut, etc.) are easily adjusted to plant gamagrass seed units in rows (Dewald et al, 2006). Row plantings can be cultivated in the early stages for weed control. Since gamagrass is a bunchgrass, established plants can have substantial bases. These can be rough to drive over with equipment. If haying is likely, consider planting in rows wide enough to minimize traffic over plants. Gamagrass stores an essential portion of its food reserves in the aboveground portion of the plant base. Reducing traffic on the plant crowns will result in less plant damage and faster re-growth. Seed units should be planted 1 to 1.5 inches deep in medium textured soil or a little deeper in light textured soil because the soil may dry out faster (Dewald, 1993). A firm seedbed is desirable for planting eastern gamagrass. Planting site preparation should be the same as for corn planting. Early corn planting season is the preferred planting time for spring plantings of gamagrass. Weed control for establishment: The time during establishment is the most critical period for weed control. Annual grasses, particularly, can cause problems in the establishment year. It is best to delay fertilization with nitrogen until the stand is established. If gamagrass is planted in rows, cultivation can be used to control weeds in the early years of establishment. Few herbicides are labeled for eastern gamagrass, although several are in development. In various studies, gamagrass has shown excellent tolerance to carryover from some of today’s commonly used corn herbicides. Some current information on herbicides is available (Roberts & Kallenbach, 1999). Weed Control After the Establishment Year: In Missouri, after the establishment year, eastern gamagrass will compete effectively with most weeds without any weed control other than proper grazing management and spring burning (Roberts & Kallenbach, 2006). Fields may be burned to control woody plants, reduce leaf diseases, improve grazing distribution, and stimulate new growth. The optimum time to burn is in the spring when the new gamagrass green-growth is about 1 inch long. Paul Salon, NRCS agronomist, states, “In the Northeast grazing management and burning alone will not provide adequate weed control.” Herbicides containing glyphosate can be applied in early spring before desirable perennial grasses break dormancy and initiate green growth. Late fall application can be made after desirable perennial grasses have reached dormancy. For broadleaf weed control, 2,4-D and dicamba containing herbicides are options; follow manufacturers label for pasture and hayland. Please contact your local agricultural extension specialist or county weed specialist to learn what works best in your area and how to use it safely. Always read label and safety instructions for each control method. Trade names and control measures appear in this document only to provide specific information. USDA NRCS does not guarantee or warranty the products and control methods named, and other products may be equally effective.
Management
Forage / Grazing & Hay: Experience by livestock grazers over the past 150 years has shown that eastern gamagrass will be eradicated from pastures unless careful controlled grazing practices are followed (Dewald et al., 2006). Gamagrass is so palatable to livestock that it is one of the first grasses to be eliminated under continuous grazing. Gamagrass re-growth has been measured at a rate of 2 inches per day. This new re-growth is tender, nutritious and greatly preferred by livestock compared to older forage. This leads to spot grazing with the same plants being defoliated almost daily, resulting in reduced plant vigor and eventual death. Eastern gamagrass stores its carbohydrates and nitrogen for re-growth in the above ground base of its stems. Therefore, grazing must be controlled so as to maintain a minimum 6 to 8 inch stubble height for all plants. But under actual grazing, the average stubble height must be considerably higher than 6 to 8 inches in order to maintain a minimum stubble height of 6 to 8 inches on most plants in the pasture. Closer grazing or clipping will reduce plant vigor and eventually reduce the stand. Short duration, high intensity rotation grazing programs that limit the cattle to 4 to 6 days per pasture with at least 8 pastures will give each pasture a 28 to 42 day rest period to recuperate. Rest periods of 40 to 45 days appear optimize the combination of stand maintenance, plant vigor, forage yield and quality. A 45-day rest period prior to killing frost is recommended for gamagrass in order to maintain stand health. Clipping gamagrass to a height of less than 6 to 8 inches will deplete the stores for re-growth and damage the stand (Gillen et al., 2006). Information from several studies indicate that clipping intervals of 40 to 45 days, beginning either at 40 to 45 days after spring green-up or at the boot stage, will optimize hay yield and quality, stand vigor and longevity. After gamagrass reaches the boot stage, forage and hay quality decreases rapidly as the plant matures. Eastern gamagrass hay production in high rainfall climates requires skillful management because hay quality is very dependent on the cutting time. Fertilization: A reliable, general guideline for eastern gamagrass fertilization is to follow the recommendations for the fertilization of corn for silage (Roberts & Kallenbach, 2006). This is particularly true for lime, phosphorous and potassium. Applications of these amendments should be made according to soil test recommendations. Soil should be tested before planting and both phosphorous and potassium applied at planting to reach a medium test level for corn. The optimum soil pH range for gamagrass is probably from 5.4 to 6.0. Another estimate of the soil pH range is a minimum of 5.1 (Foy, 1999) and a maximum of 7.5 (Sharp Brothers Seed Company, 1999). Nitrogen is more efficient when the total seasonal application is split across the hay and grazing season. For example, the initial nitrogen application is made at gamagrass green-up and additional nitrogen applied following each cutting or grazing period. Studies suggest that the optimum level for nitrogen fertilizer is between 200 and 300 lb N per acre per year, and a lower level may be optimum in regions with less than 35 inches of annual precipitation and in northern regions (Brakie, 1998; Brejda et al., 1996; Brejda et al., 1997; Douglas et al., 2002). Growers should monitor the N, P and K requirements of irrigated eastern gamagrass and apply nutrients to meet the need of each harvest.
Pests and Potential Problems
A Missouri farmer sent photographs of eastern gamagrass to the Plant Diagnostic Clinic of the University of Missouri. These photographs depicted plants with dead and diseased leaves and crowns that appeared dead or rotted. Barb Corwin, diagnostic clinic director, concluded that the disease was “take-all” (Gaeumannomyces graminis) (Clubine, 2001). The take-all fungus persists in soil and crop residues. The fungus is usually more severe under high moisture conditions, high soil pH, and low soil nitrogen. The soil pH level in the field with the take-all infected plants was 6.5. Two years before the take-all fungus was identified on eastern gamagrass, a commercial seed producer in Clifton Hill, Missouri, noticed take-all like symptoms on gamagrass. The producer contacted Chet Dewald, researcher at the USDA-ARS Southern Plains
Range
Research Station in Woodward, Oklahoma. While not acknowledging a name for the disease, Chet Dewald suggested burning the gamagrass residue in the spring and applying nitrogen fertilizer. The producer followed these suggestions and the disease symptoms disappeared. The suggested management practices to control take-all in eastern gamagrass are to apply adequate nitrogen fertility, leave good height residuals 45 days before frost, and burn the gamagrass residue every second or third spring. Two viruses, “sugarcane mosaic virus strain maize dwarf mosaic virus B” and “maize dwarf mosaic virus” can infect eastern gamagrass (Seifers et al., 1993). Both viruses are transmitted by aphids. These viruses can infect, and then over-winter on eastern gamagrass. Piper et al. (1996) describes the disease symptoms, “The disease symptoms caused by these viruses vary from a general mosaic to oblong chlorotic and necrotic spots throughout the leaf. The mosaic symptoms can vary from a pale green, very mild mosaic to a very distinct, bright mosaic on the entire plant, and are most apparent in early to mid-season as the plants emerge from dormancy.” Severe levels of the diseases caused by these viruses can reduce gamagrass growth and yield. Larva of the southern corn stalk borer [Diatraea crambidoides (Grote)] has been identified in the crown tissue of eastern gamagrass (Krizek et al., 2002b). The authors state, “This pest occurs from Delaware/Maryland to Florida and the inland states (KS, OH, OK, MS, and AZ). This pest feeds upon corn, grain sorghum, and Johnsongrass and attacks eastern gamagrass. The best strategy for avoiding damage to eastern gamagrass from the southern corn stalk borer is to remove thatch from the crop before winter. Farmers should be alert for this pest because it is highly destructive and can result in severe losses in yield of eastern gamagrass. Through rigorous management practices to prevent accumulation of thatch on the fields, it is possible to minimize the pest problem.” Eastern gamagrass plants infested with the maize billbug [Sphenophorus maidis (Chittenden)] were found in a germplasm nursery at Woodward, OK and in a 12-year-old grazed, gamagrass pasture at Fort Supply, OK (Maas et al., 2003). The authors state, “Damage infected during the life cycle of the pest will have a negative impact on seed production from the loss of reproductive tillers. Maize billbug damage may also contribute to the rate of center “die-out” of the crowns of eastern gamagrass. The control measures used in corn production may not be effective for eastern gamagrass. Research is needed to characterize the life cycle of maize billbug in eastern gamagrass and to determine effective methods of control.” Springer et al. (2004) estimated the potential loss in eastern gamagrass forage yield due to the combined infestation of both the southern corn stalk borer and maize billbug. The estimate of economic loss in eastern gamagrass hay production was from about $3.00 to $23.00, with an average of $11.00 per acre. These estimates are based on a $40.00 per ton price for gamagrass hay. The authors state, “Unless the damage to eastern gamagrass plants was severe the cost of chemical control would be greater than the return from the forage and it is not known if chemical control would be effective…. An integrated approach to their control is likely the best. The first step is to develop eastern gamagrass cultivars with resistance to these pests…. Management strategies might include using trap crops, changing harvest dates to remove forage before insects bore into culms, or late fall burning or grazing to kill some larva and remove thatch from plants which makes insects more vulnerable to freezing.” The eastern gamagrass cultivars Highlander, Jackson, and Pete were compared in replicated entry trials for either one or two years (2001-2002) at three locations (Coffeeville, Prairie and Raymond, MS) (Grabowski et al., 2003). None of the plants of Jackson survived after 2001 at Coffeeville. These plants showed signs of damage from disease symptoms caused by Pythium species and Rhizoctonia species. Ergot was observed on spikes of Highlander eastern gamagrass in Mississippi (J. Grabowski, personal communication, 2005). Although it has not been positively identified, this ergot is believed to be Claviceps tripsaci, which has previously been reported on eastern gamagrass seedheads (Hardison, 1953).
Seeds and Plant Production
Plant Production , Use soil moisture sensors to measure the soil moisture of Tripsacum dactyloides (L.) L. var. occidentale Cutler & Anders..
Plant Production
Researchers at the USDA-ARS Southern Plains Range Research Station in Woodward, Oklahoma recommend planting gamagrass for seed production in rows 40 to 48 inches wide, with 4 to 6 seed units planted per foot (Dewald et al., 2006). The affect of several agronomic parameters on eastern gamagrass seed unit production was examined in a study conducted in central Iowa (Lemke et al., 2003). The parameters were cultivar, Pete and Iuka IV, applied nitrogen (0, 50, 100, 200 lb nitrogen per acre), seed unit harvest time, and defoliation, which was designed to stimulate grazing. The application of 50 lb nitrogen per acre significantly increased the number of seed units in the second year of the study (P<0.05). However, the number of seed units did not increase at nitrogen amounts above this level. In fact, an increase in nitrogen from 100 to 200 lb per acre decreased seed unit numbers. The harvest time that optimized the number of seed units collected was approximately 2 weeks after the terminal spikes began shattering. The authors state, “If seed and livestock producers with eastern gamagrass stands are willing to accept moderate seed yield reductions, they have the option of managing stands for both forage and seed production.” A suggested seeding density for the cultivar Highlander is 9 to 19 lb seed units/acre with a minimum row width of 36 inches when the planting will be used for seed production (J. Grabowski, personal communication, 2005). Nitrogen had a minimal effect on seed yield, grain weight, percent seed fill and seed germination. Results of this study suggest that seed producers of Highlander eastern gamagrass in the upper southeastern states should apply nitrogen fertilizer in a single application of 50 to 75 lb/acre when spring re-growth reaches 10 inches. Environmental influences and timing of harvest were critical factors impacting seed yield and quality of Highlander. Eastern gamagrass terminal spikes occur on the top of the stem, and lateral spikes occur at the leaf axil, which is the angle between the leaf and stem. Seed units on the terminal spikes mature earlier than seed units on the lateral spikes. Field observations suggest that a good indictor of the optimum harvest time for Highlander eastern gamagrass is when the anthers of approximately 75% of the flowers on the lateral spikes have shed their pollen (Grabowski, personal communication, 2005). An increase in the percentage of gamagrass seed units that contained a caryopsis was obtained by partitioning seed units using either an air fractionating aspirator (Carter-Day, Model No. GF 21, Minneapolis, MN) or a gravity separator (Oliver MFG, Rocky Ford, CO) following the cleaning of gamagrass seed units with an air seed cleaner (Clipper M2B. A.T. Ferrell and Co., Saginaw, MI) (Douglas et al., 2000b). Krizek et al., (2000) showed that 21 days at a constant temperature of 86 ºF is sufficient time for conducting eastern gamagrass germination tests. Cultivars, Improved, and Selected Materials (and area of origin) The eastern gamagrass cultivar Pete was developed at the USDA, NRCS Plant Materials Center in Manhattan, Kansas (Fine et al.,1990; USDA-NRCSa, 2006). Pete closely resembles the wild strains of eastern gamagrass in Kansas and Oklahoma. It is non-uniform and widely adapted. The expected area of use of Pete includes the eastern third of Nebraska, the eastern halves of Kansas and Oklahoma, and the adjacent areas of Arkansas, Iowa, and Missouri. It can be grown farther west on irrigated and sub-irrigated sites. Pete has been established successfully in southern New York. Pete is marginally adapted to selected sites in southeastern South Dakota and southern Minnesota (USDA Hardiness Zone 4b). The eastern gamagrass cultivar Iuka IV was developed by the USDA, ARS Southern Plains Range Research Station in Woodward, Oklahoma (Dewald et. al., 2006). Farmers and ranchers have established Iuka IV in the Great Plains from southern Texas (30th parallel north) to southern Nebraska and eastern New Mexico. Also, farmers and ranchers have established Iuka IV in the region from Iowa to Maryland and southward to Louisiana. The eastern gamagrass cultivar Jackson was developed by the USDA, NRCS Plant Materials Center in Nacogdoches, Texas (USDA-NRCSb, 2006). Seed of Jackson was collected in 1986 from a native stand in Jackson County, Texas. Little variation from seed is anticipated because Jackson is an apomictic tetraploid and is therefore genetically stable. The expected area of use of Jackson extends from the eastern half of Texas though the southeastern states excluding Florida. The eastern gamagrass selected class of natural germplasm San Marcos was developed by the USDA, NRCS James E. ‘Bud’ Smith Plant Materials Center in Knox City, Texas (USDA-NRCSc, 2006). Seed of the San Marcos germplasm was originally collected from plants located in Hays County, Texas, near the town of San Marcos. Average annual precipitation for the collection area is about 33 inches. San Marcos may be used in monocultures for pasture and hay or as a component in seed mixtures for range plantings. It is anticipated that San Marcos will be adapted to the following NRCS Major Land Resources Areas (MLRA) in central Texas and southern Oklahoma: 78B, 78C, 78D, 80A, 80B, 81B, 81C, 82, 83A, 84B, 84C, 85, 86A, 86B, 87A, and 87B. Sam Marcos is adapted to a wide range of soil types, but will perform best on sandy loams, clay loams and clays. It is well adapted to low, moist sub-irrigated sites. It is productive in areas with annual precipitation less than 28 inches only if supplied with supplemental irrigation. The eastern gamagrass cultivar Highlander was developed jointly by the USDA, NRCS Jamie L. Whitten Plant Materials Center in Coffeeville, Mississippi, the USDA, NRCS Jimmy Carter Plant Materials Center in Americus, Georgia and the Mississippi Agricultural and Forestry Experiment Station, Mississippi State, Mississippi (Grabowski et al., 2005). Highlander was collected in 1990 in Montgomery Country, Tennessee (MLRA 122); average annual precipitation for this location is 1016 millimeters (40 inches). Little variation from seed is anticipated because the Highlander is an apomictic tetraploid and is therefore genetically stable. Highlander is recommended for hay production. It is best used as a hay crop; however it can be grazed if managed to prevent damage to the plant stand (i.e. rotational grazing). Highlander is well adapted for use in the eastern portions of USDA Hardiness Zones 6b to 8a, using Interstate 35 as its western limit. The eastern gamagrass cultivar Medina was developed by the USDA, NRCS Plant Materials Center in Nacogdoches, Texas (USDA-NRCSb, 2006). Medina seed was originally collected in1986 from a native stand in the Hondo Creek bottom of Median County, Texas. Medina is a versatile cultivar with potential for forage uses that include pasture, hay, green chop, and silage. Medina, without irrigation, is adapted to areas that receive 25 or more inches of annual rainfall throughout USDA Hardiness Zones 8 to 9 (excluding Florida). Medina is adapted to many soil types, but deep sandy soils are not suitable. The eastern gamagrass cultivar Bumpers was developed by the USDA, NRCS Plant Materials Center in Booneville, Arkansas (USDA-NRCSe, 2006). The original seed of Bumpers was collected from a native roadside stand in Yell County, Arkansas (MLRA 118). Bumpers is useful for livestock grazing. Other forage options are for perennial hay, silage, and green chop. Bumpers is well adapted for use in the mid-south portions of USDA Hardiness Zones 6a through 8a and for rainfall areas of 40 to 60 inches. This cultivar is recommended for western Arkansas, southern Missouri and eastern Oklahoma.. The eastern gamagrass selected class of natural germplasm St Lucia, and selected class of natural germplasm Martin were developed by the USDA NRCS Plant Materials Center in Brooksville Florida (USDA-NRCSd, 2006). The original germplasm of St. Lucia was vegetatively collected from St. Lucia County, Florida. The original germplasm of Martin was vegetatively collected from Martin County, Florida. Both St. Lucia and Martin were selected for use as an ornamental landscape plant in xeriscape and for use in buffer strips. Both St. Lucia and Martin are diploid (2n = 2x = 36) and will outcross, producing progeny with varying color characteristics. Vegetative propagation is necessary to maintain the blue foliage characteristic of both St. Lucia and Martin. Both St. Lucia and Martin are adapted to USDA Hardiness Zones 8 to 10, but they will not survive temperatures below 0 °F for extended periods of time. The eastern gamagrass cultivar Verl was released in 2005 by the USDA, ARS, in cooperation with the Oklahoma Agricultural Experiment Station and the USDA, NRCS, (Springer et al., 2006). Verl is a fertile triploid (2n = 3x = 54) that reproduces predominately by apomixis. Verl is recommended for pasture and hay in eastern and southern United States. Verl has excellent seed production. At Woodward, OK, Verl produced an equivalent seed yield of 152 lb/acre. Verl is susceptible to feeding damage from the maize billbug and the southern cornstalk borer. Infestation by these insects reduces seed production of eastern gamagrass. Verl may be susceptible to Rhizoctonia, Pythium, and Bipolaris species. Cultivars comprised of northern ecotypes, such a Pete and Iuka IV, initiate growth earlier in the spring than cultivars comprised of ecotypes from the southern portion of the range such as Jackson and Highlander. Malcome Kirkland NRCS agronomist, suggests that farmers can lengthen the gamagrass grazing season, in the southern region of the gamagrass range, by utilizing both northern and southern ecotype cultivars. The northern ecotype cultivars will provide more forage early in the spring and the southern ecotype cultivars more forage during middle and late summer. According to NRCS agronomist Sharon Pfaff, native Florida ecotypes are not dormant during winter. Therefore, this strategy would be ineffective in Florida. This strategy may also prove ineffective in the northern portion of the gamagrass range because many southern ecotypes are not winter hardy in this region. Contact your local Natural Resources
Conservation
Service (formerly Soil Conservation Service) office for more information. Look in the phone book under ”United States Government.” The Natural Resources Conservation Service will be listed under the subheading “Department of Agriculture.” A comprehensive treatment of eastern gamagrass that includes cultivar forage yield information is available from: Eastern Gamagrass (Tripsacum dactyloides): A Plant for Forage, Conservation and Bioenergy. URL: http://npdc.usda.gov/publications/index.html
References
Aiken, G. 1997. Temporal effects on steer performance and nutritive values for eastern gamagrass grazed continuously for different durations. J. Anim. Sci. 75: 803-808. URL: http://jas.fass.org/ Ahring, R. M. & Frank, H. 1968. Establishment of eastern gamagrass from seed and vegetative propagation. J. Range Manage. 21: 27-30 Anderson, R. C. 1985. Aspects of the germination ecology and biomass proportion of eastern gamagrass (Tripsacum dactyloides L.). Bot. Gaz. 146: 353-364. Brakie, M. R. 1998. Yield and quality of eastern gamagrass selections as affected by clipping interval and N rates. M.S. Thesis, Stephen F. Austin State University, Nacogdoches. Brejda, J. J., Brown, J. R., Lorenz, T. E. & Henry, J. 1996. Eastern gamagrass responses to different harvest intervals and nitrogen rates in northern Missouri. J. Prod. Agric. 9: 130-135. Brejda, J. J., Brown, J. R., Lorenz, T. E., Henry, J. & Lowry, S. R. 1997. Variation in eastern gamagrass forage yield with environments, harvests, and nitrogen levels. Agron. J. 89: 702-706.
Forage
and Grassland Council: 333-336. Staver, K. W. 2000. Production and nutrient uptake by native warm-season grasses. Pp. 314-317 in: Dickerson, J. A, Ritchie, C. A. Ritchie, J. C. (eds.), Proc. 2nd Eastern Native Grass Symposium, Baltimore. Tharel, L. M. 2000. Nutrient utilization and dry matter production of eastern gamagrass, switchgrass and Old World bluestem. Pp. 29-31 in: Eastern Gamagrass Technology Update. USDA-NRCS Plant Materials Program. USDA-NRCSa, 2006. Plant Materials Center at Manhattan, KS. URL: http://plant-materials.nrcs.usda.gov/centers/ USDA-NRCSb, 2006. Plant Materials Center at Nacogdoches, TX. URL: http://plant-materials.nrcs.usda.gov/centers/ USDA-NRCSc, 2006. Plant Materials Center at Knox City, TX. URL: http://plant-materials.nrcs.usda.gov/centers/ USDA-NRCSd, 2006. Plant Materials Center at Brooksville, FL. URL: http://plant-materials.nrcs.usda.gov/centers/ USDA-NRCSe, 2006. Plant Materials Center at Booneville, AR. URL: http://plant-materials.nrcs.usda.gov/centers/ USDA-NRCS-NPDC. 2007. Eastern gamagrass (Tripsacum dactyloides): A plant for forage, conservation and bioenergy. URL: http://npdc.usda.gov/publications/index.html; accessed 13AUG07.
Fact Sheet
Uses
The primary uses of eastern gamagrass are for producing hay and haylage. It is more productive, palatable, and nutritious than the other native perennial warm season grasses. Gamagrass may be used as an alternative to annual silage crops, or as a pasture forage where intensive rotational grazing is well managed.
Status
Please consult the PLANTS Web site and your State Department of Natural Resources for this plant’s current status (e.g. threatened or endangered species, state noxious status, and wetland indicator values).
Description
Tripsacum dactyloides L., eastern gamagrass, is a native, warm season, perennial, sod-forming grass that is a distant relative of corn. This plant can reach a height of up to 8 feet. Seed is produced from June to September. The seed heads are 6 to 10 inches long and are made up of one to several spikes. The leaves can be 3/8 to 3/4 inch wide and 12 to 24 inches long. They also have a well defined midrib.
Adaptation
Gamagrass is native to the eastern half of the United States, It does best in moderately well drained to somewhat poorly drained soils, Lowlands, subirrigated, or irrigated areas that are not alkaline will also support this species well, Use soil moisture sensors to measure the soil moisture of Tripsacum dactyloides (L.) L. var. occidentale Cutler & Anders.., It will tolerate extended periods of flooding, For a current distribution map, please consult the Plant Profile page for this species on the PLANTS Website,
Establishment
Site selection must be made carefully before planting. Gamagrass may be seeded with conventional equipment into a thoroughly prepared seedbed if the site has good weed control history. Sites formerly in cool season pastures or hayland should be sown without tillage to avoid exposing weed seeds to good germination conditions. The seeds must first be stratified (exposed to cold, wet conditions) for 8 weeks before spring sowing. Seed may be purchased stratified from commercial growers. To stratify artificially, place the seeds in a burlap bag until the bag is about 1/2 full. Soak this in a 1% solution of fungicide for 10-12 hours. Afterwards, drain the seeds and seal them, along with the sack, in a plastic bag. Store them this way for 8 weeks at 35-45 °F. Stratification may also be achieved by planting in the fall after November 1, but before frost is in the soil. The planting site must also be prepared. Do this as you would for corn. Planting should be done when the soil is at least 55 °F; early corn planting season is preferred. If planting gamagrass with corn, plant the corn first and sow the grass between the corn rows. In the Northeast, gamagrass is prone to frost heaving over the first winter after planting on some soils. The use of moderately well drained or well drained soils makes this possibility less likely. On soils with somewhat poor drainage, use all strategies to produce the largest possible plants by the end of the first
Plant Traits
Growth Requirements
| Cold Stratification Required | No |
|---|---|
| Hedge Tolerance | None |
| Hedge Tolerance | Low |
| Frost Free Days, Minimum | 170 |
| Frost Free Days, Minimum | 140 |
| Frost Free Days, Minimum | 140 |
| Fire Tolerance | High |
| Fire Tolerance | High |
| Fertility Requirement | High |
| Fertility Requirement | High |
| Fertility Requirement | High |
| Drought Tolerance | Medium |
| Drought Tolerance | Low |
| Drought Tolerance | Low |
| Cold Stratification Required | Yes |
| Cold Stratification Required | Yes |
| Temperature, Minimum (°F) | -23 |
| CaCO3 Tolerance | None |
| CaCO3 Tolerance | None |
| CaCO3 Tolerance | Low |
| Anaerobic Tolerance | Medium |
| Anaerobic Tolerance | Low |
| Anaerobic Tolerance | Low |
| Adapted to Medium Textured Soils | Yes |
| Adapted to Medium Textured Soils | Yes |
| Adapted to Medium Textured Soils | Yes |
| Adapted to Fine Textured Soils | Yes |
| Adapted to Fine Textured Soils | Yes |
| Adapted to Fine Textured Soils | Yes |
| Adapted to Coarse Textured Soils | Yes |
| Adapted to Coarse Textured Soils | Yes |
| Adapted to Coarse Textured Soils | No |
| Moisture Use | High |
| Temperature, Minimum (°F) | -23 |
| Temperature, Minimum (°F) | -23 |
| Shade Tolerance | Intolerant |
| Shade Tolerance | Intolerant |
| Shade Tolerance | Intolerant |
| Salinity Tolerance | None |
| Salinity Tolerance | None |
| Salinity Tolerance | None |
| Root Depth, Minimum (inches) | 20 |
| Root Depth, Minimum (inches) | 20 |
| Root Depth, Minimum (inches) | 20 |
| Precipitation, Minimum | 16 |
| Precipitation, Minimum | 16 |
| Precipitation, Minimum | 16 |
| Precipitation, Maximum | 60 |
| Precipitation, Maximum | 50 |
| Hedge Tolerance | None |
| Moisture Use | High |
| Moisture Use | Medium |
| pH, Maximum | 7.5 |
| pH, Maximum | 7.5 |
| pH, Maximum | 7.5 |
| pH, Minimum | 5.1 |
| pH, Minimum | 5.1 |
| pH, Minimum | 5.7 |
| Planting Density per Acre, Maxim | 2700 |
| Planting Density per Acre, Maxim | 2700 |
| Planting Density per Acre, Minim | 1700 |
| Planting Density per Acre, Minim | 1700 |
| Precipitation, Maximum | 60 |
Morphology/Physiology
| Active Growth Period | Spring and Summer |
|---|---|
| Toxicity | None |
| Toxicity | None |
| Toxicity | None |
| Shape and Orientation | Erect |
| Shape and Orientation | Erect |
| Shape and Orientation | Erect |
| Resprout Ability | No |
| Flower Conspicuous | No |
| Fire Resistant | No |
| Fire Resistant | No |
| Fire Resistant | No |
| Flower Color | Yellow |
| Flower Color | Yellow |
| Flower Color | Yellow |
| Flower Conspicuous | No |
| Flower Conspicuous | No |
| Fall Conspicuous | No |
| Foliage Color | Green |
| Foliage Color | Green |
| Foliage Color | Green |
| Foliage Porosity Summer | Dense |
| Foliage Porosity Summer | Dense |
| Foliage Porosity Summer | Dense |
| Foliage Porosity Winter | Moderate |
| Foliage Porosity Winter | Moderate |
| Bloat | None |
| Resprout Ability | No |
| Active Growth Period | Spring and Summer |
| Active Growth Period | Spring and Summer |
| After Harvest Regrowth Rate | Moderate |
| After Harvest Regrowth Rate | Rapid |
| After Harvest Regrowth Rate | Rapid |
| Bloat | None |
| Bloat | None |
| Resprout Ability | No |
| C:N Ratio | Low |
| C:N Ratio | Low |
| C:N Ratio | Medium |
| Coppice Potential | No |
| Coppice Potential | No |
| Coppice Potential | No |
| Fall Conspicuous | No |
| Fall Conspicuous | No |
| Lifespan | Long |
| Height, Mature (feet) | 6.0 |
| Known Allelopath | No |
| Known Allelopath | No |
| Known Allelopath | No |
| Leaf Retention | No |
| Leaf Retention | No |
| Leaf Retention | No |
| Lifespan | Long |
| Foliage Texture | Coarse |
| Lifespan | Long |
| Low Growing Grass | No |
| Low Growing Grass | No |
| Low Growing Grass | No |
| Nitrogen Fixation | None |
| Nitrogen Fixation | None |
| Nitrogen Fixation | None |
| Height, Mature (feet) | 5.0 |
| Height, Mature (feet) | 5.0 |
| Foliage Porosity Winter | Moderate |
| Foliage Texture | Coarse |
| Foliage Texture | Medium |
| Fruit/Seed Color | Brown |
| Fruit/Seed Color | Brown |
| Fruit/Seed Color | Brown |
| Fruit/Seed Conspicuous | No |
| Fruit/Seed Conspicuous | No |
| Growth Form | Bunch |
| Growth Form | Bunch |
| Growth Form | Bunch |
| Growth Rate | Moderate |
| Growth Rate | Rapid |
| Growth Rate | Rapid |
| Fruit/Seed Conspicuous | No |
Reproduction
| Seed per Pound | 7200 |
|---|---|
| Propagated by Tubers | No |
| Propagated by Tubers | No |
| Propagated by Tubers | No |
| Propagated by Sprigs | Yes |
| Propagated by Sprigs | Yes |
| Propagated by Sprigs | No |
| Propagated by Sod | No |
| Propagated by Sod | No |
| Propagated by Sod | No |
| Propagated by Seed | Yes |
| Propagated by Seed | Yes |
| Propagated by Seed | Yes |
| Propagated by Cuttings | No |
| Propagated by Cuttings | No |
| Fruit/Seed Period End | Summer |
| Seed per Pound | 7300 |
| Seed per Pound | 7500 |
| Seed Spread Rate | Slow |
| Seed Spread Rate | Slow |
| Seed Spread Rate | Slow |
| Seedling Vigor | Low |
| Seedling Vigor | Low |
| Seedling Vigor | Medium |
| Small Grain | No |
| Small Grain | No |
| Small Grain | No |
| Vegetative Spread Rate | Moderate |
| Vegetative Spread Rate | Moderate |
| Vegetative Spread Rate | Slow |
| Propagated by Corm | No |
| Propagated by Cuttings | No |
| Bloom Period | Early Summer |
| Bloom Period | Early Summer |
| Bloom Period | Late Spring |
| Commercial Availability | Routinely Available |
| Commercial Availability | Routinely Available |
| Commercial Availability | Routinely Available |
| Fruit/Seed Abundance | Low |
| Fruit/Seed Abundance | Low |
| Fruit/Seed Abundance | Low |
| Fruit/Seed Period Begin | Spring |
| Fruit/Seed Period Begin | Summer |
| Fruit/Seed Period Begin | Summer |
| Fruit/Seed Period End | Fall |
| Fruit/Seed Period End | Fall |
| Fruit/Seed Persistence | No |
| Fruit/Seed Persistence | No |
| Propagated by Corm | No |
| Propagated by Corm | No |
| Propagated by Container | No |
| Propagated by Container | No |
| Propagated by Container | No |
| Propagated by Bulb | No |
| Propagated by Bulb | No |
| Propagated by Bulb | No |
| Propagated by Bare Root | No |
| Propagated by Bare Root | No |
| Propagated by Bare Root | No |
| Fruit/Seed Persistence | No |
Suitability/Use
| Palatable Browse Animal | High |
|---|---|
| Palatable Graze Animal | High |
| Palatable Graze Animal | High |
| Palatable Graze Animal | High |
| Palatable Human | No |
| Palatable Human | No |
| Palatable Human | No |
| Post Product | No |
| Post Product | No |
| Post Product | No |
| Protein Potential | High |
| Protein Potential | High |
| Protein Potential | High |
| Pulpwood Product | No |
| Pulpwood Product | No |
| Pulpwood Product | No |
| Veneer Product | No |
| Veneer Product | No |
| Veneer Product | No |
| Lumber Product | No |
| Berry/Nut/Seed Product | No |
| Berry/Nut/Seed Product | No |
| Berry/Nut/Seed Product | No |
| Christmas Tree Product | No |
| Christmas Tree Product | No |
| Christmas Tree Product | No |
| Fodder Product | Yes |
| Fodder Product | Yes |
| Fodder Product | Yes |
| Palatable Browse Animal | High |
| Lumber Product | No |
| Lumber Product | No |
| Naval Store Product | No |
| Naval Store Product | No |
| Naval Store Product | No |
| Nursery Stock Product | No |
| Nursery Stock Product | No |
| Nursery Stock Product | No |
| Palatable Browse Animal | High |