Sitka Alder
Scientific Name: Alnus viridis (Chaix) DC. ssp. sinuata (Regel) Á. Löve & D. Löve
| General Information | |
|---|---|
| Usda Symbol | ALVIS |
| Group | Dicot |
| Life Cycle | Perennial |
| Growth Habits | ShrubTree, |
| Native Locations | ALVIS |
Plant Guide
Alternate Names
Green alder, mountain alder, wavy-leaf alder, slide alder, Alnus alnobetula, Alnus crispa ssp. laciniata, Alnus crispa ssp. sinuata, Alnus sinuata, Alnus viridis var. sinuata, and Duschekia sinuata (USDA NRCS, 2011a).
Uses
Erosion control and reclamation: Sitka alder is a valuable shrub for slope and stream bank stabilization and general erosion control on disturbed, nutrient poor sites. It can be planted for acid, coal, placer, and copper mine spoil reclamation, soil enrichment, and other land rehabilitation efforts where an easy to establish, deciduous shrub is desired (Uchytil, 1989). The species may also be useful in hedgerows, the shrub row of field windbreaks, and other conservation buffers. Forestry: As host to symbiotic nitrogen fixing bacteria in its roots, Sitka alder is particularly important for improving forest site productivity and is sometimes used as a companion or nurse shrub in conifer plantations (Delong and Sanborn, 2000; Hauessler et al., 1990). Compared to red alder (Alnus rubra), Sitka alder is considered potentially less competitive with young conifers because of its smaller stature and slower growth (Harrington and Deal, 1982). It is not used for commercial timber but is a source of firewood. Wildlife: The palatability of Sitka alder is considered poor and forage value low for most big game animals and livestock (Uchytil, 1989), but others report that it is one of the most palatable of the native alders, being rated fair to good as browse for sheep in some areas (USDA Forest Service, 1988). Selective browsing of this species by moose occurs in Idaho during summer months as leaves remain green (Pierce, 1984). It is also considered high-quality moose browse in British Columbia. Elk will browse the tender young shoots, while white-tailed and mule deer feed on leaves and twigs (Haeussler et al., 1990). Caribou also browse on Sitka alder. Alder twigs and leaves are consumed by muskrats, rabbits, snowshoe hares, and squirrels, while the seeds, buds, or catkins are an important source of food in winter for numerous song and game birds (Haeussler et al., 1990; Healy and Gill, 1974; Martin et al., 1951). Beavers eat the bark and use the stems to build lodges and dams (USDA Forest Service, 1988). Thickets provide thermal and hiding cover for big game and other wildlife, as well as nesting habitat for many small birds (Uchytil, 1989). Sitka alder is a source of pollen for honeybees, native bees, and other insects during the spring. Ethnobotanic: Native peoples of North American had practical uses for Sitka alder or its close relative green alder (Alnus viridis ssp. crispa). The bark was a source of red or brown dye which was used to color wool and tanned animal skins (Agar and Agar, 1980; Anderson, 1939; Kari, 1985; Rousseau, 1945; Turner et al., 1980). Documentation of alder being used as a fragrance or scent (Turner et al., 1990) may refer to Sitka alder which is known for being sticky and sweet smelling. Alder was burned as firewood and preferred for smoking fish (Ager and Agar, 1980; Kari, 1985). One or both alders were considered a sign of water and the hard wood was used for making snowshoes, bows, or spoons (Compton, 1993; Kari, 1985; Turner et al., 1990). Sitka and green alder had several medicinal uses. Pistillate catkins of Sitka alder were crushed and eaten raw for treating gonorrhea (Compton, 1993). 2 The inner bark or ointments made from it were used to treat skin problems such as wounds, skin ulcers, and swellings. Fresh scraped bark juice was applied to the skin to relieve itching from rash and a fresh infusion was made to treat poison ivy. The bark was also used for treating constipation, jaundice, and diarrhea. Leaf decoctions were used to treat burns and swollen wounds. Alder roots are high in tannins and were boiled and drunk as an astringent (Ellis et al., 1995). A decoction of stems was apparently drunk as a remedy for colds or dried stems were placed in the nose or chewed for the same reason (Turner, et al., 1990). The catkins of Sitka alder can be eaten raw or cooked but have a bitter taste (Plants for a Future, 2011). Apparently, young buds and inner bark are edible as well, but the bark will cause vomiting if not dried or aged first (Elias et al., 1995; Plants for a Future, 2011). Figure 2. Mature shrub row of Sitka alder at Corvallis, OR. Photo by Dale Darris.
Status
Please consult the PLANTS Web site and your State Department of Natural Resources for this plant’s current status (e.g., threatened or endangered species, state noxious status, and wetland indicator values).
Description
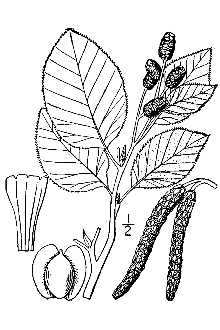
General: Sitka alder (Betulaceae family) is a deciduous shrub or small tree that grows to a height of 1-6 m (3-19 ft) in the mountains and 4-12 m (13-39ft) at lower elevations. Initially fast growing, its habit is freely branching at the base, upright to bent, and multi-stemmed with a rounded, open crown. In the wild, crooked or leaning specimens are usually the result of avalanches, slides, or snowpress. Life expectancy is 25 to 50 years under typical growing conditions (Elias, 1980). The flowers are monecious with separate male and female catkins (aments; apetalous unisexual flowers) on the same plant. The staminate (male) inflorescence is naked (borne without bud scales), forms late in the growing season, opens the following spring, and turns from yellow-green to brown. When fully expanded they are slender, 8-14 cm (3.0-5.5 in) long, and drooping. Immature pistillate (female) catkins form by midsummer, remain enclosed within bud scales during winter, emerge the following spring, and occur in clusters of 3 to 6. At flowering they are 0.7-1.0 cm (0.3-0.4 in) long, later becoming woody, cone-like structures (strobili or strobiles) comprised of many leathery or woody scales. Mature cones are 1.3-2.0 cm (0.5-0.8 in) long and half as thick, ovate to ovoid-ellipsoid in shape, and born on a slender peduncle. The fruit is a small nutlet (seed) with thin membranous wings that are twice the width of the seed (Elias, 1980; Hickman, 1993; Hitchcock et al., 1969). Unlike other alders, the catkins of Sitka alder open with the formation of the leaves as opposed to beforehand. Flowering occurs in April, May, or June depending on elevation and latitude. Natural pollination is by wind. This species produces seed primarily through outcrossing, but self pollination is possible as evidenced from controlled pollinations at Corvallis, OR. The cone and its seeds mature between mid-September and December (Harrington et al., 2008). Figure 3. Sitka alder male catkins (below leaves) and female catkins (above leaves) in flower (April). Photo by Dale Darris The branches are slender, glabrous, light brown to reddish brown or grey, and slightly zigzagged in appearance. The older bark is thin and smooth grey to blue-grey in color. Twigs are reddish to yellow brown, at first pubescent later becoming smooth with conspicuous lenticels (pores in the bark). The winter buds are sessile on new growth, 1.2-1.4 cm long (0.5-.06 in), sharply acute, and covered with 3 to 6 brownish-red to dark purple overlapping scales (Elias, 1980; Hickman, 1993; Hitchcock et al., 1969, Oregon State University, 2011). 3 Figure 4. Sketch of Sitka alder plant parts. Reprinted with permission, University of Washington Press. Leaves are alternate and the blades thin, sticky and fragrant when young, narrowly to broadly ovate, 7-14 cm (2.8-5.5 in) long, and 3-10 cm (1.2-4.0 in) wide. They have a fine single or double serrate-denticulate to sinuate margin. The leaf surfaces are glabrous except for hairs along the major vein axils, yellow-green above, and slightly paler and shiny beneath. The base of the leaf is rounded to subcordate while the tip is acute to slightly acuminate. The petiole is glabrous, 1.3-1.9 cm (0.5-0.8 in) long and grooved on the upper surface (Elias, 1980; Hickman, 1993; Hitchcock et al., 1969; Oregon State University, 2011). The roots of Sitka alder form a highly fibrous system that aids in soil erosion control. They also develop beneficial symbiotic relationships with both ectomycorrhizal fungi and actinobacteria (actinomycetes) in the genus Frankia. The latter association results in the formation of root nodules which are active sites for fixation of atmospheric nitrogen. Contributions to soil nitrogen by Sitka alder in the Pacific Northwest have been estimated at 20 to 150 kg N/ha (16-122 lbs/ac) per year (Binkley, 1986). Distribution: In North America, Sitka alder occurs from southern and western Alaska and the Yukon southward to northern California and eastward to southwestern Alberta, western Montana, northwestern Wyoming, and Idaho. It is one of three subspecies of Alnus viridis that form a circumpolar group distributed across northern North America, Asia, and Europe. The other subspecies are A. viridis ssp. crispa and A. viridis ssp. fruticosa. While Sitka alder can be found anywhere from sea level to above timberline (0-2745 m, 0-9000 ft), its most common occurrence is above 900 m (3000 ft) in the mountains (Elias, 1980; Hickman, 1993; Oregon State University, 2011; USDA Forest Service, 1988). It is rare to infrequent below 100 m (325 ft) in Oregon, Washington, and southern British Columbia. Sitka alder freely interbreeds with green alder (A. viridis ssp. fruticosa) and intermediate types can be found in British Columbia (Hauessler et al., 1990). For most current distribution, please consult the Plant Profile page for this species on the PLANTS Web site. Figure 5. Distribution map for Sitka alder in North America. Reproduced with permission, Flora of North American Association. Habitat: Sitka alder is a thicket forming, pioneer, early-seral, and mid-seral species. It is well recognized as an ecologically important species for natural colonization and stabilization of landslide chutes, steep slopes, rock slides, stream banks, areas of flood deposition and scour, and exposed mineral soils following glacial retreat, avalanches, road building, logging, fire, soil slumping, and other drastic disturbances. Typical habitat for Sitka alder includes moist montane woods, rocky or sandy coasts (Alaska), stream banks, bogs, fens, lakeshores, moist talus slopes, the edges of moist meadows, and the north face of rocky outcrops or other shady aspects (Elias, 1980; Hauessler et al., 1990; Mitchell, 1968, Uchytil, 1989). The species is usually found in full sun, but has intermediate shade tolerance and can persist under a forest canopy (DeLong and Sanborn, 2000; Hauessler et al. 1990; Uchytil, 1989). Because of its wide occurrence, Sitka alder is associated with many woody plant communities. In the Cascades and Rocky Mountains, it is found within subalpine fir (Abies lasiocarpa), Pacific silver fir (Abies amabilis), western hemlock (Tsuga heterophylla) and western redcedar (Thuja plicata) forests (Uchytil, 1989). In Alaska, Sitka alder ranges 4 from low elevation wetland habitats where it may dominate or form the tall shrub layer in Lutz spruce (Picea x lutzii) forests (Gracz et al., 2008), to subalpine habitats where it can also form extensive stands or be found intermixed with herbaceous community types (Mitchell, 1968). In interior British Columbia, Sitka alder is a common shrub component of lodgepole pine (Pinus contorta) communities (Delong and Sanborn, 2000). Figure 6. Thicket of Sitka alder with crooked stem at Talapus Lake, Cascade Mountains, WA, July 1991. ©Susan McDougall @ USDA-NRCS PLANTS Database. Adaptation: Sitka alder grows on soils that vary from infertile mineral to rich humus covered substrates, highly acidic to neutral pH (3.3-7.5), and coarse to medium texture (rocky, gravelly, loamy sands, sandy loams, silts, loams) (Hauessler et al., 1990; Mitchell, 1968). It also does well in heavier clay loam soils that are nutritionally poor but moist (Plants for a Future, 2011). Sitka alder can strongly influence the soil characteristics of the site it occupies. Because of nitrogen fixation within the root nodules, sites with established Sitka alder colonies generally have higher available soil nitrogen than adjacent plant communities (Haeussler and Coates, 1986; Hauessler et al., 1990). Its abundant, nitrogen-rich leaf litter is also an important source of organic matter for soil building and nutrient cycling (Uchytil, 1989). The species also produces an acidifying effect on the soil (Hauessler et al., 1990). In some regions, Sitka alder and red alder occupy the same areas. However, Sitka alder is more likely to be found on steep sites and those with well drained, rocky or coarse textured substrates while red alder occurs on swampy areas, moist floodplains, and poorly drained soils (Batzli and Dawson, 1997). While reported to be indicative of high water tables (Uchytil, 1989) and found along ponds, swamps, and other wet areas (Ellis, 1980), Sitka alder appears more maladapted to flooding compared to red alder. Unlike red alder, Sitka alder lost substantial root and shoot biomass and did not restore growth during flooding (20 days) or recovery periods (10 days) (Batzli and Dawson, 1997). Others report that “Sitka alder prefers moist, relatively well drained soils but will grow on sites ranging from submesic to subhygric or possibly hygric.” [Submesic = water removed readily in relation to supply and available for moderately short periods following precipitation; hygric = water removed slowly enough to keep the soil wet for most of the growing season. Permanent seepage and mottling are present (Walmsley et al., 1980)]. However, it’s not abundant on very wet sites with high water tables. The species is poorly adapted to drought (Haeussler et al., 1990). Natural regeneration: The seed of Sitka alder can travel long distances by wind or water (Uchytil, 1989). Germination occurs between early spring and June on moist, exposed mineral soils following natural disturbances as well as logging and heavy burning. In the wild, Sitka alder also reproduces vegetatively by sprouting from damaged root collars or stumps. Shoots can also form where roots are exposed in streams (Hauessler et al., 1990).
Establishment
Like other alders, Sitka alder can be established by direct seeding revegetation and reclamation sites, but ideal seeding rates are unknown. Physiological dormancy can be present in seed lots (Baskin and Baskin, 2002). Therefore, it is advisable for dry untreated seed to be planted in the fall on moist mineral seedbeds for best results. Seed should be covered with a thin layer of soil, mulch, or peat. Suggested sowing depths are similar to those used for other alders grown in bare-root nursery beds (2-5 mm, 0.1-0.2 in) (Harrington et al., 2008). Results from Sitka alder seeding can be mixed and poor germination rates have been reported (Delong and Sanborn, 2000). If spring sowing of Sitka alder is preferred, it is suggested that stored seed be cold moist stratified (moist chilled) just prior to planting. However, the ideal length of time for chilling varies among reports. Seed dormancy for Sitka alder may differ by provenance, population, seed moisture content, storage history, or age of seed as it does with other alders (Harrington et al., 2008). Dry seed of Sitka alder can germinate at higher rates or more uniformly if it undergoes cold moist stratification for one to three months at 1-3ºC (34-38ºF) (Emery, 1988; Wick et al., 2008). Others report improved results with cold stratification periods for green or Sitka alder of 14 days (Farmer et al., 1985), six (3-9) weeks (Forestry Commission, 2011), one month (McLean, 1967), or nine weeks (Darris et al., 1994). Pregermination seed treatments with fungicide (captan) and peroxide treatments may have a 5 beneficial effect by reducing pathogens present on the seed coat (Harrington et al., 2008). However, fungicide treatment can also reduce germination (Darris et al., 1994). Others soak the seed for 24 hours prior to sowing and indicate no chilling treatment is required (Hudson and Carlson, 1998). Fresh seed can lack dormancy and may germinate without treatment if sown immediately on the soil surface or at a very shallow depth. Sitka alder can readily establish in the field using seedlings. Roots should be inoculated with Frankia bacteria by the producer and if possible, with an appropriate ectomycorrhizal fungus prior to or at outplanting. Stock type is typically container, but bare-root material in the range of 45-60 cm (18-24 in) tall may be used as well. Indications of seedling quality such as high root to shoot ratio, the presence of root nodules, and stem caliper are as or more important than height. Proper seedling selection, care, and handling, site preparation, planting techniques, vegetation management, and animal damage control measures generally described for establishing other woody plants should be observed (Darris, 2001; Elefritz et al., 1998; Hallman, 1993; Nolte and Otto, 1996; Rose and Morgan, 2000). The best time to plant Sitka alder varies somewhat by region. In western Oregon and western Washington, fall and early winter are preferred. Bare-root material is usually planted in late winter or early spring when stock is most available. For riparian buffer plantings or stream bank stabilization along low velocity streams, a suggested overall spacing of 1.2 m (4 ft) is common (Voss, 1997). For effective erosion control on critical slopes and steep banks, a narrower spacing (0.6 m x 0.6 m, 2 ft x 2 ft, minimum 3 rows deep) similar to that used for smooth alder (Alnus serrulata) seedlings is advised (USDA NRCS, 2007). Plantings are also done in a grid fashion or clumps of three to five seedlings each. Recommended within-row spacing for windbreaks of medium to large sized shrubs like Sitka alder is 1.2 m (4 ft) for single rows and 1.8 m (6 ft) for multiple rows (USDA Soil
Conservation
Service, 1991).
Management
Like most tree and shrub plantings, a monitoring and maintenance program for Sitka alder can be critical for successful establishment and growth. The survival and condition of outplanted stock should be surveyed at least annually for the first three to five years. Remedial measures may include replanting, weed control, and animal damage prevention. Specific guidelines for windbreak maintenance are described elsewhere. In forestry, one objective is to achieve balance between the benefits of nitrogen fixation and associated long term site productivity afforded by Sitka alder, and its competitive interactions with crop trees that reduce their growth (Simard and Nicholson, 1990). Strategies include interplanting of alder and pine (Pinus spp.) at compatible densities or selectively removing or retaining established alder in young conifer plantations (Delong and Sandborn, 2000). Some suggest species like Douglas-fir (Pseudotsuga menziesii) and Ponderosa pine (Pinus ponderosa) be planted several (2-6) years in advance of Sitka alder (Uchytil, 1989). The effect of alder density and brush cutting or removal on conifer growth is the subject of research and recommended densities or percent cover will vary by goal, climatic-vegetation zone, soil moisture regime, site productivity, timber species, and other factors. For instance, in the sub-boreal spruce (Picea spp.) zone of Canada, low priority should be given to controlling Sitka alder on submesic sites with lodgepole pine unless the density of alder is uniformly high (i.e., >45%) (Brockley and Sanborn, 2003). In another report, moderate levels of Sitka alder did not appear to inhibit diameter growth of lodgepole pine or spruce. Results suggest that on mesic sites in the dry alder areas, brushing of Sitka alder is unnecessary for the release of lodgepole pine. Also, brushing is ineffective at reducing growth- limiting factors to spruce on wet alder sites (Simard et al., 2004).
Pests and Potential Problems
Sitka alder is susceptible to many of the same pathogens as red alder (Hepting, 1971). Fungal pathogens include leaf spots, Septoria spot, branch and trunk cankers, heart rot, and powdery mildew. Leaf spot and mildew diseases may show up in seedling stock. In red alder, top-kill caused by Bortrytis spp. and stem cankers caused by Septoria alnifolia can cause significant loss in nursery yields, requiring multiple applications of fungicide during the growing season (Ahrens et al., 1992). Alders in general can be host to a number of insect pests including aphids, scales, borers, sawflies, and leaf miners (Furniss and Carolin, 1980, Johnson and Lyon, 1991). Other common pests of Sitka alder are the alder leaf beetle (Altica ambiens), poplar-willow borer (Cryptorhynchus lapathi), and western tent caterpillar (Malacosoma californicum) (Hauessler et al., 1990). Several insect pests were observed on seedlings or mature shrubs growing at Corvallis, OR. The black vine root weevil (Otiorhynchus sulcatus) occasionally proved troublesome during containerized nursery production of Sitka alder. 6 The grub-like larvae feed on roots while adult beetles create a notched appearance on the margin of the leaves. Insecticidal soil drenches or parasitic nematodes can be useful in managing root weevil larvae, while approved foliar applied insecticides may be necessary for adult weevil control. Timing is critical in order to match treatment with the insect’s life cycle (DeAngelis and Garth, 1993; Rosetta, 2010). Oyster shell scale (Lepidosaphes ulmi) and poplar-willow borer were two other pests noted in the mature specimens at Corvallis. Scales can be treated with a dormant oil spray. Borers are controlled by cutting and destroying infested limbs. Always read label and safety instructions for each pest control method. Trade names and control measures appear in this document only to provide specific information. USDA NRCS does not guarantee or warranty the products and control methods named, and other products may be equally effective.
Environmental Concerns
Concerns
Concerns
Alders produce considerable windborne pollen with allergenic activity. Sitka alder pollen is classified as a moderate allergen and like other alders, may have cross-reactivity with pollen from other plants (e.g. birch and hazel). The result can be hay fever (allergic rhinitis) and atopic dermatitis in humans (Smith et al., 2007). Livestock poisoning from plant parts has not been reported. Sitka alder can increase after overstory tree removal and readily volunteer on disturbed sites such as recently logged and burned over forestland. Therefore, in some reforestation efforts it is considered a minor to major competitor with conifer tree seedlings and an obstacle for plantation establishment and growth (Coates et al., 1990; Hauessler et al., 1990, Uchytil, 1989). Colonization along roadsides and utility right-of-ways may also necessitate control.
Control
Brushing is sometimes used to set back or control Sitka alder in conifer stands and right-of-ways when density or growth becomes too high, Use soil moisture sensors to measure the soil moisture of Sitka Alder., Brushing is defined as the “removal of undesirable herbaceous and woody vegetation by manual or mechanical means” (Canadian Forest Service, 1995), The species can sprout new shoots after cutting so more than one treatment may be needed for conifer release (B,C, Ministry of Forests, 1997), For Alnus viridis, July and August cutting results in the least sprouting and lowest height of new growth (Harvey et al,, 1998), Other methods of control alone or in combination with cutting can be effective, Foliar applied herbicides such as glyphosate, triclopyr, and 2,4-D can give good control (B,C, Ministry of Forests, 1997; Coates et al,, 1990), Triclopyr can also be used for stump and basal bark treatments, Hack and squirt (herbicide) and girdling methods are inappropriate because of Sitka alder’s multi-stemmed growth habit (B,C, Ministry of Forests, 1997), A native fungal pathogen (Chondrostereum purpureum) has been approved in Canada as a biological control alternative to the use of glyphosate and triclopyr herbicides, The agent is applied as a paste to re-sprouting stumps of red and Sitka alder following manual cutting (Health Canada, 2007), Moderate burns are listed as a control treatment (Coates et al,, 1990), but the species can also be favored by fire (Hauessler et al,, 1990), Brown (using glyphosate) and burn treatments are effective (Coates et al,, 1990), Sheep grazing is not suggested because Sitka alder is not effectively browsed (B,C, Ministry of Forests, 1997), Please contact your local forester, extension specialist, or county weed specialist to learn what treatments are legal and work best in your area and how to use them safely, Always read label and safety instructions for each control method, Trade names and control measures appear in this document only to provide specific information, USDA NRCS does not guarantee or warranty the products and control methods named, and other products may be equally effective,
Seed and Plant Production
Plant Production
Plant Production
Seed production: Sitka alder seed can be obtained from natural stands or seed orchards. The species commences flowering and producing seed at an age of four (6) to seven (8) years (Hauessler et al., 1990; Uchytil, 1989). However, populations grown under cultivation at Corvallis, OR, flowered as early as age three. Plants will produce seed every year with a bumper crop occurring every three to five years (Hauessler et al., 1990). Seed orchards are best established in full sun on moist, well drained sites with coarse to medium textured soils that are mildly acidic. Selections should be properly isolated from other subspecies and populations of both Alnus viridis and Alnus crispa. For seed certification of native and naturalized plants in Oregon, the standard minimum distance apart for cross pollinated species including Sitka alder is 275 m (900 ft). Summer irrigation beyond the establishment year may be desirable on dryer sites especially if plants are not mulched. Insect and disease pests should be monitored and if necessary treated with approved integrated pest management (IPM) methods as required, according to label and safety instructions. Methods for collection, extraction, and storage of red alder seed are generally applicable to Sitka alder. The seed is collected in fall or early winter when the 7 female cones (strobiles) turn brown and scales begin to open. Seed may also be sufficiently mature if the cones are turning brown in color and their scales easily separate by twisting the cone at the top and bottom (Hibbs and Ager, 1989). They can be hand harvested or the branches flailed over a tarp. The cones are thoroughly dried at ambient temperatures by suspending them in fine mesh bags or placing them on elevated screens. Some of the seed will fall out during drying with the remainder removed by tumbling or shaking. Red alder and presumably Sitka alder cones can also be kiln dried at 16-27°C (60-80°F) for two to seven days for quicker extraction (Hibbs and Ager, 1989). Seed can be cleaned with an air screen machine and processed through an air column to remove small extraneous particles (Harrington et al., 2008). At the NRCS Corvallis Plant Materials Center (PMC), 95 cones yielded 27 g (~1 oz.) of clean seed. Data on seeds per pound vary widely, ranging from 1,467,000 seeds/kg (666,000 seeds/lb) (USDA NRCS, 2011b) to 3,740,000 seeds/kg (1,700,000 seeds/lb). Differences may be genetic, environmental, or related to the amount of good versus empty seed which is hard to determine. Seed of Alnus viridis is classified as orthodox meaning it can be collected and stored dry with little loss of viability (Forestry Commission, 2011). When Sitka alder seed was stored in paper envelopes and refrigerated at 3-5°C (37-41°F) at Corvallis, OR, it maintained a germination rate of over 50% after three years. Red alder seed has been preserved for longer periods (10-20 years) without substantial loss in viability when dried to less than 10% moisture content and frozen in moisture proof containers at minus 12ºC (10°F) (Hibbs and Ager, 1989). Appropriate conditions for storage of Alnus viridis seeds are 8 to 10 percent moisture content at less than 4°C (39°F) (Forestry Commission, 2011). Vegetative propagation: Hardwood cuttings of Sitka alder did not root in one study (Java and Everett, 1992), but others report success with spring or fall planted hardwood cuttings on wet sites (DeLong and Sandborn, 2000). Rooting success has been reported with green stem cuttings (Carpenter et al., 1984). Cuttings were dipped in a solution of 2000 ppm IBA (indole-3-butyric acid) and dusted with a mixture of Rootone 10 rooting compound and fungicide. The rooting medium was sterile perlite and vermiculite (1:1). They were then misted intermittently for 10 weeks and fertilized weekly with a liquid fertilizer during the last month. Results improved by applying bottom heat of 21°C (70°F). Others report some success rooting cuttings that are taken just after the leaves fall and planted outdoors in a sandy soil (Plants for a Future, 2011). At Corvallis, OR, summer wood cuttings made in July and treated with hormones were unsuccessful. Field grafting has been reported for certain cultivars of alder, but the process is generally difficult for this genus. The Corvallis PMC experimented with a device for hot-callusing graft unions of dormant stock but was unsuccessful (Darris et al., 1994). The species has been propagated successfully using tissue culture (Tremblay and Lalonde, 1984). Container production: Sitka alder is readily adapted to container nursery production (Hudson and Carlson, 1998; Wick et al., 2008) both in a greenhouse and outdoor beds. Plants grown in larger containers have better outplanting performance (Harrington et al., 2008). The use of standard growing media that are 75 to 100 % peat, moderate to well drained, and amended with micronutrients and slow release fertilizer is suggested. Unless fall sown, the use of stratified seed is recommended. Given the requirement of light for germination, seed should be surface sown or covered with a very thin layer of silica sand in pots. Germination usually occurs within three weeks (Corvallis PMC propagation notes; Wick et al., 2008). Depending on the original container size, seedlings may be re-potted into 1- gallon or larger pots within a period of 12 to 18 weeks. Container plants can be maintained in a shadehouse with periodic fertilization and irrigation. Some growers apply a balanced soluble fertilizer one or more times in the spring and early summer. A fall hardening phase may mean discontinuation or reduction of fertilizer and a reduction of water as early as August. Pruning, if needed, is done one or more times on soft tissue before mid-August (Hudson and Carlson, 1998). Seedling inoculation: For optimal growth and improved root nodulation, young seedlings or growing media should be inoculated with the appropriate Frankia actinobacteria using cultured isolates or dilute slurry made from water and macerated nodules (Ahrens, 1994; Quoreshi et al., 2007; Subramaniam et al., 1991). Superior cultures can be raised on a special medium, harvested by centrifugation, homogenized, suspended in distilled water and applied to four-week-old seedlings as a soil drench (Subramaniam et al., 1991). Others suggest the inoculum be mixed with a carrier like peat and incorporated into the potting media or nursery beds (Martin et al., 1985). It has been demonstrated that container grown green alder can be successfully inoculated with Frankia under commercial nursery conditions without changing normal operational schedules. Unfortunately, commercial sources of inoculum are generally unavailable (Quoreshi et al., 2007). 8 Frankia can be obtained from fresh nodules growing on the roots of donor plants, though this method is less reliable and the extracted strain would be unknown. The nodules are rinsed thoroughly with tap water, then crushed, ground, or macerated, homogenized in a blender, and stored as slurry in a refrigerator for a short period until used. The homogenate may be filtered through muslin, diluted with water, and then applied to containerized seedlings at four weeks or a seedbed prior to sowing (Wheeler et al., 1991; Ahrens et al., 1992). Soil obtained beneath the canopy of existing Sitka alder stands or from a makeshift inoculum bed of older, nodulated seedlings is a third source of Frankia. Work at the Corvallis PMC confirmed that a thin band of this soil (dried and sifted) placed in the container prior to sowing, coupled with periodic application of a balanced liquid fertilizer or incorporation of a slow-release fertilizer, can produce the most vigorous seedlings (Darris et al., 1994). Low to moderate rates of nitrogen fertilization promote the formation of the nitrogen-fixing nodules (Harrington et al., 2008). Sitka alder will also respond positively to inoculation with certain species of beneficial ectomycorrhizal fungi (Hudson and Carlson, 1998). Procedures for precise fungal inoculation of the root system are described elsewhere (Castellano and Molina, 1989). Companies also produce and sell fungal associates that may function well with Sitka alder. The manufacturer’s experts should be consulted. In some instances, soil borrowed from existing stands of Sitka alder may suffice at outplanting. Bare-root production: Sitka alder has been successfully produced as 1+0 and plug+1 bare-root stock in outdoor nursery beds. [1+0 (1-0) stock are 1- year old seedlings grown in one location before lifting. Plug+1 (P+1, P-1) stock are seedlings started in small cavity containers then transplanted after six months to a year into a nursery bed where they are grown for another year]. Seedlings grown in fumigated beds have benefited from inoculation with Frankia just prior to seeding. While alder species can be successfully fall seeded, most nurseries sow in the spring (Harrington et al., 2008). Presumably treated (stratified) seed is used for improved germination. Open bed seedling densities of 60-180 seedlings/m2 (5-15/ft2) recommended for other alders should also work for Sitka alder. Management of top growth, timing (sowing, lifting, etc.) and grading of seedlings will likely differ by alder species, climate, and nursery. However, general guidelines for nursery culture including methods of bed inoculation with Frankia, sowing, fertilization, irrigation, pest management, transplanting, and storage developed for the production of red alder (Ahrens, 1994; Ahrens et al., 1992; Harrington et al., 2008) are adaptable to Sitka alder. Cultivars, Improved, and Selected Materials (and area of origin) Skamania Germplasm is a selected class pre-variety of Sitka alder that originates from a natural stand growing above the north shore of the Columbia River, in the vicinity of Beacon Rock in Skamania County, WA. It was released in 2006 by the USDA Natural Resources Conservation Service, Corvallis, OR, in cooperation with the Agricultural Experiment Stations of Oregon and Washington. While this species of alder is more common at mid to sub-alpine elevations, Skamania Germplasm represents a high quality seed source from, and for use at, lower elevation. Recommended applications include stream bank stabilization, reclamation of disturbed lands, soil improvement of forestlands, and conservation buffers (windbreaks, hedgerows). Suggested area of use is western Washington and western Oregon below an elevation of 457 m (1500 ft) (Darris et al., 2002). Figure 7. Bare-root nursery bed of Skamania Germplasm Sitka alder seedlings at former Washington DNR Nursery, Bow, WA. Photo by Dale Darris. Genetic differences among seed sources or provenances of Sitka alder have been demonstrated. A study in British Columbia with 28 populations of Sitka alder showed clear geographic patterns in frost hardiness, dry weight, growth, and germination parameters that related to latitude and distance from the coast (Benowicz et al., 2000). The differences are probably adaptive (Centre for Forest Gene Conservation, 2011). At present, there are no published seed zones or seed transfer guidelines for this species. In such cases, some suggest US EPA Level III ecoregions can be used as surrogate seed transfer zones (Withrow-Robinson and Johnson, 2006). As with most endemic plant species, careful attention should be paid to the natural origin of the seed or nursery stock (longitude, latitude, elevation, soil, and climate) and how well it is projected to perform at the planting site. Sitka alder seedlings are 9 produced by a number of nurseries in the western US and Canada.
References
Ahrens, G.R., A. Dobkowski, and D.E. Hibbs. 1992. Red alder: guidelines for successful regeneration. Special publication 24. Forest Research Laboratory, Oregon State University, Corvallis, OR. Ahrens, G.R. 1994. Seedling quality and nursery practices for red alder. In: D.E. Hibbs, D.S. DeBell, and R.F Tarrant (editors). The Biology and Management of Red Alder. Oregon State University Press, Corvallis, OR. Agar, T.A. and L.P. Agar. 1980. Ethnobotany of the Eskimos of Nelson Island, Alaska. Arctic Anthropology 27:26-48. Anderson, J. P. 1939. Plants used by the Eskimo of the Northern Bering Sea and Arctic Regions of Alaska. Am. J. Bot. 26:714-16. B.C. Ministry of Forests. 1997. Operational summary for vegetation management dry alder complex. Forest Practices Branch, Ministry of Forests, Province of British Columbia, Victoria, BC. Baskin, C.C. and J.M. Baskin. 2002.
Propagation
protocol for production of container Alnus viridis (Chaix) DC plants; University of Kentucky, Lexington, Kentucky. In: Native Plant Network. [http://www.nativeplantnetwork.org (accessed 30 August 2011)]. Moscow (ID): University of Idaho, College of Natural Resources, Forest Research Nursery. Batzli, J. McCray and J.O. Dawson. 1997. Physiological and morphological responses of red alder and Sitka alder to flooding. Physiologia Plantarum 99: 653-663. Benowicz, A., Y.A. El-Kassaby, R.D. Guy, and C.C. Ying. 2000. Sitka alder (Alnus sinuata Rydb.) genetic diversity in germination, frost hardiness, and growth attributes. Silvae Genetica 49: 206-212. Binkley, D. 1986. Forest nutrition management. Wiley, New York. Brockley, R.P. and P. Sanborn. 2003. Effects of Sitka alder on the growth and foliar nutrition of young lodgepole pine in the central interior of British Columbia. Can. J. For. Res. 33: 1761-1771. Canadian Forest Service. 1995. Silvicultural terms in Canada. Second Edition. Policy, Economics and International Affairs Directorate. Natural Resources Canada. Canadian Forest Service. Ottawa, ON. Carpenter, C.V., L.R. Robertson, J.C. Gordon, and D.A. Perry. 1984. The effect of four new Frankia isolates on growth and nitrogenase activity in clones of Alnus rubra and Alnus sinuata. Can. J. For. Res. 14:701-706. Castellano, M.A. and R. Molina. 1989. Mychorrhizae. In: T.D. Landis, R.W. Tinus, S.E. McDonald, and J.P. Barnett. The Container Tree Nursery Manual. Volume 5. Agriculture Handbook 674. USDA Forest Service, Washington D.C. Centre for Forest Conservation Genetics. 2011. Sitka alder (Alnus viridis (Chaix.) D. C.) University of British Columbia, Vancouver, BC. [http://genetics.forestry.ubc.ca/cfcg/proj_cataloguing/s_alnuvir.html] Coates, D., S. Haeussler, and J. Mather. 1990. A guide to the response of common plants in British Columbia to management treatments. FRDA Handbook 008. British Columbia Ministry of Forests, Victoria, BC. Compton, B.D. 1993. Upper North Wakashan and Southern Tsimshian ethnobotany: the knowledge and usage of plants and fungi among the Oweekeno, Hanaksiala (Kitlope and Kemano), Haisla (Kitamaat) and Kitasoo peoples of the central and north coasts of British Columbia. Ph.D. Dissertation, University of British Columbia. Vancouver, BC. Darris, D.C. 2001. Tips for planting trees and shrubs: revegetation and landscaping. Plant Materials Technical Note No. 3. USDA Natural Resources Conservation Service, Portland, OR. Darris, D.C, T.R. Flessner, and J.D. Conrod Trindle. 1994. Corvallis Plant Materials Center Technical Report: plant materials for streambank stabilization 1980-1992. USDA Natural Resources Conservation Service, Portland, OR. Darris, D.C, R. Tracey, and S. Lambert. 2002. Fact sheet on Skamania Germplasm Sitka alder. USDA-NRCS, Plant Materials Center, Corvallis, OR. DeAngelis, J. and G. Garth. 1993. Management of root weevils in the nursery and landscape. The Digger. Oregon Association of Nurseries, Wilsonville, OR. DeLong, C. and P. Sanborn. 2000. Management of Sitka alder and willows: a strategy to minimize loss of habitat and maximize benefit to long term soil productivity. Forest Research Note No. PG-22. Forest Resources and Practices Team, Ministry of Forests, Prince George, BC. Elefritz, M, M.M. Atkinson, and S.A. Fitzgerald. 1998. The care and planting of tree seedlings on your woodland. EC 1504. Oregon State University Extension Service, Corvallis, OR. Elias, T.S. 1980. The complete trees of North America. Book Division, Times Mirror Magazines, Inc. (publisher), Van Nostrand Reinhold Company, New York (distributor). Elias S., E. Ottoni, N. Verma, D. Kreuger, S. Roburn, and C. Wulff. 1995. An ethnobotany of the UBC Arboretum. University of British Columbia, Vancouver, B.C. Adapted for the web 1998. [http://www.botany.ubc.ca/facilities/arboretum] 10 Emery, D.E. 1988. Seed propagation of native California plants. Santa Barbara Botanic Garden, Santa Barbara, CA. Farmer, R.E., M.L. Maley, M.U. Stoehr, and F. Schnedenburger. 1985. Reproductive characteristics of green alder in northwestern Ontario. Can. J. Bot. 63: 2243-2247. Forestry Commission. 2011. Seed storage and pre-treatment for Alnus viridis. Great Britain. [http://www.forestry.gov.uk/fr/INFD-7FAB3P] Furniss, R.L. and V.M. Carolin. 1980. Western forest insects. Miscellaneous publication No. 1339. USDA Forest Service. U.S. Government Printing Office, Washington, D.C. Gracz, M. et al. 2008. Wetland classification of the Kenai Peninsula lowlands, Alaska. Kenai Watershed Forum. Fritz Creek, AK. The Alaska Natural Heritage Program, University of Alaska, Anchorage, AK. [http://www.kenaiwetlands.net/plant_community_classification_i.htm] Hallman, R. 1993. Reforestation equipment. 9324-2837-MTDC. Technology and Development Program, USDA Forest Service, Missoula, MT. Harrington, C.A. and R.L. Deal. 1982. Sitka alder, a candidate for mixed stands. Can. J. For. Res. 12 (1): 108-111. Harrington et al. 2008. Alnus P. Mill. alder. In: F.T. Bonner and R.P. Karrfalt (editors). The Woody Plant Seed Manual. Agriculture Handbook 727. USDA Forest Service, Washington, D.C. Harvey, E.M., F.W. Bell, and J.O. Boateng. 1998. Manual, motor–manual and mechanical brushing tools for conifer release. Northwest Science and Technology Technical Note TN–42. In: F.W. Bell, M. McLaughlan and J. Kerley (compilers). Vegetation Management Alternatives – A Guide to Opportunities. Ontario Ministry of Natural Resources, Thunder Bay, ON. Hauessler, S. and D. Coates. 1986. Autecological characteristics of selected species that compete with conifers in British Columbia: a literature review. Land Management Report No. 33. Ministry of Forests, Information Services Branch, Victoria, BC. Hauessler, S., D. Coates, and J. Mather. 1990. Autecology of common plants in British Columbia: a literature review. FRDA Report 158. Forestry Canada and B.C. Ministry of Forests, Victoria, BC. Health Canada. 2007. Registration decision. Chondrostereum purpureum strain PFC2139 Cp-PFC2139 Chontrol Paste. Pest Management Regulatory Agency, Ottawa, ON. Healy, W.M. and J.D. Gill. 1974. Alders. In: J.D. Gill and W.M. Healy (compilers). Shrubs and vines for Northeastern Wildlife. General Technical Report NE-9.USDA Forest Service. Northeastern Forest Experimental Station, Upper Darby, PA. Hepting, G.H. 1971. Diseases of forest and shade trees of the United States. Agriculture Handbook 386. USDA Forest Service. U.S. Government Printing Office, Washington D.C. Hibbs, D.E. and A.A. Ager. 1989. Red alder: guidelines for seed collection, handling and storage. Special Publication 18. Forest Research Laboratory, Oregon State University, Corvallis, OR. Hickman, J.C. (editor). 1993. The Jepson Manual, higher plants of California. University of California Press. Berkeley, Los Angeles, and London. Hitchcock, C.L., et al. 1969. Vascular Plants of the Pacific Northwest. Part 2: salicaceae to saxifragaceae. University of Washington Press. Seattle and London. Hudson, S. and M. Carlson. 1998. Propagation of interior British Columbia native plants from seed. British Columbia Ministry of Forests, Research Program. Kalamalka Forestry Centre, Vernon, BC. Java, B.J. and R.L. Everett. 1992. Rooting hardwood cuttings of Sitka alder and thinleaf alder. In: W.P. Cleary, E.D. McArthur, D. Bedunah, and C.L. Wambolt (compilers). Proceedings-Symposium on Ecology and Management of Riparian Shrub Communities, Sun Valley, ID, May 1991. USDA Forest Service. Intermountain Research Station, Ogden, UT. Johnson, W.T. and H.H. Lyon. 1991. Insects that feed on trees and shrubs. Comstock Publishing Associates, Cornell University Press, Ithaca, NY, and London. Martin, K.J., Y. Tanaka, and D.D. Myrold. 1991. Peat carrier increases inoculation success with Frankia on red alder (Alnus rubra Bong.) in fumigated nursery beds. New Forests 5: 43-50. Martin, A.C., H.S. Zim, and A.L. Nelson. 1951. American wildlife and plants: a guide to wildlife food habits. Dover Publications, Inc. New York. McLean, A. 1967. Germination of forest range species from southern British Columbia. J.
Fact Sheet
Uses
Sitka alder is a valuable species for stabilizing drastically disturbed, nutrient poor sites such as eroded streambanks, landslide chutes, steep rocky slopes, areas of flood deposition and scour, and exposed mineral soils following glacial retreat, avalanches, and massive soil slumping. It is also suitable for reclaiming acid, coal, and copper mine spoils and other soil enriching, revegetation efforts where a nitrogen fixing shrub (via bacteria in its root nodules) is desired. At the proper densities, the species may also be useful in improving forest site productivity as a companion or nurse shrub in young conifer plantations. The palatability of Sitka alder is considered poor and forage value low for most ungulates, but others report that it is one of the most palatable of the native alders, especially for sheep. Moose, elk, and certain deer will browse young shoots, leaves, or twigs, as will rabbits, snowshoe hares, and squirrels. The seeds, buds, or catkins are an important source of food in winter for numerous song and game birds. Beavers eat the bark and use the stems to build lodges and dams. Thickets provide thermal and hiding cover for big game and other wildlife, as well as nesting habitat for many small birds. Native Americans used the bark and its extracts to make a red-brown dye, cure several internal ailments, and treat skin wounds, itching, and swelling, including poison oak. Leaves and roots were used medicinally as well. The wood was preferred by some tribes for smoking salmon, woodworking, and making bows, baskets, and snowshoes.
Description
Sitka alder is a deciduous shrub or small tree that grows to height of 3 to 20 ft, occasionally taller, The form is upright, multi-stemmed, and freely branching at the base with a rounded crown, Use soil moisture sensors to measure the soil moisture of Sitka Alder., Male flowers are in the form of long catkins that begin opening in late winter, Found separately on the same plant are the female flowers which are cone-like (short catkins called strobiles) and bloom in early spring when the leaves appear, The seeds are winged nutlets that mature in autumn or early winter within the egg shaped woody cones, The leaves are broadly oval, 1 to 5 in, long, shiny above, wavy and finely toothed on the margins, and slightly scented and sticky beneath when young, Nodules containing nitrogen fixing bacteria form on the strong fibrous roots, The bark is smooth and grey, Twigs form a zigzag branch pattern,
Status
Please consult the Plants Web site and your State Department of Natural Resources for this plant’s current status, such as state noxious and wetland indicator values.
Adaptation
Sitka alder is a pioneer species that prefers full sun to partial shade and soils that range from mineral to rich, humus covered substrates, acid to neutral pH (3.8 to 7.5), and coarse to medium texture (gravelly, sandy, silty, loamy). However, it will tolerate moist clay soils and sites that are nutritionally poor. This species prefers moderate to good drainage. It is less tolerant to flooding and poor drainage than red alder. Plants resprout from the crown after fire. Distribution- Sitka alder occurs naturally from central Alaska south to northern California and east to Alberta, northwest Wyoming, and western Montana. The elevation range is sea-level to 9000 ft in the mountains. Habitat includes moist montane woods, rocky or sandy coastlines and talus slopes, streambanks, lakeshores, and the north face of rocky outcrops. Limitations or environmental concerns Sitka alder is host to a number of insect pests including root weevils, flea beetles, leaf rollers, borers, sawflies, leaf minors, scales, and aphids. Pathogens include leaf spots, powdery mildew, alder top-kill, and stem cankers. Some of these diseases can show up in nursery stock. The species readily volunteers into disturbed areas, making it potentially weedy and a competitor with valuable timber trees. Toxicity for this plant has not been reported.
Plant Traits
Growth Requirements
| Temperature, Minimum (°F) | -48 |
|---|---|
| Adapted to Coarse Textured Soils | Yes |
| Adapted to Fine Textured Soils | No |
| Adapted to Medium Textured Soils | Yes |
| Anaerobic Tolerance | Low |
| CaCO3 Tolerance | Low |
| Cold Stratification Required | Yes |
| Drought Tolerance | Low |
| Fertility Requirement | Low |
| Fire Tolerance | High |
| Frost Free Days, Minimum | 90 |
| Hedge Tolerance | Low |
| Moisture Use | High |
| pH, Maximum | 7.5 |
| pH, Minimum | 5.0 |
| Planting Density per Acre, Maxim | 1200 |
| Planting Density per Acre, Minim | 430 |
| Precipitation, Maximum | 200 |
| Precipitation, Minimum | 16 |
| Root Depth, Minimum (inches) | 10 |
| Salinity Tolerance | None |
| Shade Tolerance | Intermediate |
Morphology/Physiology
| Bloat | Low |
|---|---|
| Toxicity | None |
| Resprout Ability | Yes |
| Shape and Orientation | Erect |
| Active Growth Period | Spring and Summer |
| C:N Ratio | High |
| Coppice Potential | No |
| Fall Conspicuous | Yes |
| Fire Resistant | No |
| Flower Conspicuous | No |
| Foliage Color | Yellow-Green |
| Foliage Porosity Summer | Dense |
| Foliage Porosity Winter | Porous |
| Foliage Texture | Medium |
| Fruit/Seed Conspicuous | Yes |
| Nitrogen Fixation | Low |
| Low Growing Grass | No |
| Lifespan | Long |
| Leaf Retention | No |
| Known Allelopath | No |
| Height, Mature (feet) | 16.0 |
| Height at 20 Years, Maximum (fee | 10 |
| Growth Rate | Slow |
| Growth Form | Multiple Stem |
| Fruit/Seed Color | Brown |
Reproduction
| Vegetative Spread Rate | None |
|---|---|
| Small Grain | No |
| Seedling Vigor | High |
| Seed Spread Rate | Rapid |
| Seed per Pound | 666000 |
| Fruit/Seed Persistence | No |
| Propagated by Tubers | No |
| Propagated by Sprigs | No |
| Propagated by Sod | No |
| Propagated by Seed | Yes |
| Propagated by Corm | No |
| Propagated by Cuttings | No |
| Bloom Period | Summer |
| Commercial Availability | Routinely Available |
| Fruit/Seed Abundance | High |
| Fruit/Seed Period Begin | Summer |
| Fruit/Seed Period End | Fall |
| Propagated by Bare Root | Yes |
| Propagated by Bulb | No |
| Propagated by Container | Yes |
Suitability/Use
| Veneer Product | No |
|---|---|
| Pulpwood Product | No |
| Protein Potential | Low |
| Post Product | No |
| Palatable Human | No |
| Palatable Graze Animal | Low |
| Palatable Browse Animal | Low |
| Nursery Stock Product | No |
| Naval Store Product | No |
| Lumber Product | No |
| Fuelwood Product | Medium |
| Fodder Product | No |
| Christmas Tree Product | No |
| Berry/Nut/Seed Product | No |
