Salix sessilifolia Nutt. ssp. hindsiana (Benth.) Andersson
Scientific Name: Salix sessilifolia Nutt. ssp. hindsiana (Benth.) Andersson

| General Information | |
|---|---|
| Usda Symbol | SASEH2 |
| Group | Dicot |
| Life Cycle | Perennial |
| Growth Habits | ShrubTree, |
| Native Locations | SASEH2 |
Plant Guide
Alternate Names
Sandbar willow, gray willow, narrow-leaved willow, dusky willow, pussywillow , Use soil moisture sensors to measure the soil moisture of Salix sessilifolia Nutt. ssp. hindsiana (Benth.) Andersson.
Uses
Ethnobotanic: The value of willow as the raw material necessary for the manufacture of a family's household goods cannot be over-estimated. Among the Paiute, every woman carried bundles of long, slender willow which had been scraped white, and coils of willow sapwood that she had gathered and prepared during the winter months when the leaves were gone (Wheat 1967). Willow branches are used as the warp for twined baskets and the foundation in coiled baskets. Willows are used to weave water jugs, cradles for newborn infants, hats, cooking vessels, serving bowls, trays, seed beaters, and storage baskets. Some tribes use willow roots as a sewing strand. Virtually all California tribes use willow in their baskets. Tribes which use willow, such as Salix exigua, include the Chemehuevi, Paiute, Mono, Panamint, Pviotso (Northern Paiute), Shoshoni, Bannock, Ute, Washo, Chiricahua, Jicarilla Apache, Mescalero Apache, Navajo, San Carlos Apache, Western Apache, White Mountain Apache, Havasupai, Maricopa, Yavapai, Hopi, San Juan Pueblo (Tewa), Zuni, Papago, and Pima Indians extending through the American Southwest and Mexico. In Ancestral Puebloan times, willow, along with threeleaf sumac, was the material of choice for manufacturing Native American baskets. Willow is gathered from the time the leaves fall in autumn until the buds begin to swell in spring. The year-old wands without branches are chosen, and sorted by size and length. The bark can easily be stripped off in the spring when the sap rises. Willow wands with the smallest leaf scars are split and peeled to obtain the tough, flexible sapwood used for the weft in basket weaving. Color variation is achieved by alternating peeled and unpeeled willow sticks in the warp. Ute Indians used to concoct a green dye for coloring buckskin by soaking willow leaves in hot water and then boiling the mixture to concentrate the pigment. Willow roots also have been used by others to manufacture a rose-tan dye. Alfred Brousseau © Brother Eric Vogel, St. Mary’s College @ CalPhotos The Paiute built willow-frame houses covered with mats of cattails or tules. Slender willow withes were woven into tight circular fences as protection from the wind that blew sand into eyes and food. For shade, shed roofs thatched with willows, called "willow shadows", were constructed. In the Pueblo province, coyote willow branches are employed with leaves attached for thatching roofs. Other light construction uses included the tops of storage bins or racks for aerating corn while it dried, such as one recently unearthed at prehistoric Arroyo Hondo Pueblo. A bed or sleeping bench of willow poles raised high off the ground indicated a wealthy man in the Miwok culture in California's Sierra Nevada. Willow brush was placed radically over the roof timbers of an earth lodge. Boats had eight willow ribs and a gunwale of willow pole along each side. Sweat lodges are made with willow. A women’s shinney game was played on a field similar to a football field with five-foot long, sharp willow poles. A ring of rope or string was thrown into an indent in the field and the women had to move it up the field and throw it against a goal post without touching or carrying it on the poles. Counting games are played with willow counting sticks. Ancestral Puebloans used willow wood for textile loom anchors, rods to control the weaving rhythm, and finishing needles. Bows, arrow points, pot rests, scrapers and cradle parts all were crafted from willow. In later times, Navajo made weaving sticks and arrow shafts from willow along with other straight-grained woods, and Ute Indians made snowshoe frames from dried willow branches. Matting was another early product made from willows. Other implements made from willow include fire sticks twirled as a spindle to generate enough heat to ignite a flame and what appear to be prayer sticks recovered from various archaeological sites. Willow is still used for making prayer sticks by the Zunis and doubtless by some of the Rio Grande pueblo. Inner bark was used in spring for rope in California (Murphey 1959). Aspirin is the pharmaceutical equivalent of willow bark tea, which is an effective remedy for headache, fever or sore throat. More than 2,400 years ago, the Greeks learned to use extracts of several native willow species to treat pain, gout, and other illnesses. In more recent times, in 1839, salicylic acid was isolated from wild plants and manufactured synthetically. Early salicylic acid-based products had unpleasant side effects. Sixty years later, the Bayer Company developed a derivative of salicylic acid, called it aspirin, and the rest is history. Tea made from willow leaves will cure laryngitis. Willow reduces inflammation of joints and membranes. When used as an analgesic, willow treats urethra and bladder irritation, infected wounds, and eczema. Willow is used as an over-all treatment of many diseases, including hay fever, diarrhea, prostatitis, satyriasis, and relief of ovarian pain. A poultice is made for treating gangrene and skin ulcers. For one remedy used by the Paiute, burned willow charcoal was added to water and taken as a tea to stop diarrhea. A San Juan tribal elder said he used willow leaves to make his mouth water and relieve thirst. Young willow shoots can be stripped of their bark and eaten. The inner bark can be eaten raw, prepared like spaghetti, or made into a flour. The young leaves may be eaten in case of emergency Other Uses: Ecological diversity, bank and sediment stabilization, maintenance of channel morphology, water quality improvement, ground-water recharge, flood abatement, fish and wildlife habitat, ribs of boats, and games. Riparian Ecosystem Services and Functions: The riparian zone essentially encompasses those alluvial sediment deposits where river and alluvial ground water supplement that available from local precipitation. High-to-low elevations, north-south and east-west gradients, and steep-to-shallow terrain all influence the relationship between geomorphic and fluvial processes and vegetation community structure. Riparian ecosystem functions include the following: • Ecological diversity. • Riparian vegetation traps sediments and nutrients from surface runoff and prevents them from entering the aquatic system. • Dense matrix of roots in the riparian zone can serve as an effective filter of shallow groundwater. • Water quality is improved through filtration and trapping of sediment, nutrients (particularly nitrogen dissolved in groundwater), and pollutants. • Riparian vegetation tends to prevent the river from down-cutting or cutting a straight path (channeling), thus promoting a sinuous course, ground-water recharge, and maintenance of an elevated water table. • Riparian areas act as a sponge by absorbing floodwaters which is then slowly released over a period of time, which minimizes flood damage and sustains higher base flows during late summer. • Structurally complex riparian vegetation communities provide many different habitats and support a diverse array of animal species. Different groups of animals occupy or use the different layers of vegetation, and this multi-story arrangement is often present nowhere else in the arid landscapes. • Canopies of plants growing on streambanks provide shade, cooling stream water, while roots stabilize and create overhanging banks, providing habitat for fish and other aquatic organisms. Wildlife: Rabbits and many ungulates, including deer, moose, and elk, browse on willow twigs, foliage and bark (Martin 1951). Beavers consume willow branches, while several species of birds eat willow buds and young twigs. California's riparian forests support a high diversity of breeding birds (Miller 1951). In one study conducted on the Sacramento River, 147 bird species were recorded as nesters or winter visitants’ (Laymon 1985). The percentage of breeding individuals that are migratory is very high in the cottonwood-willow habitat. Moister conditions in the cottonwood-willow forest may promote lusher plant growth, higher invertebrate populations and, therefore, more available food for flycatchers, warblers and other migratory, insectivorous birds. Riparian areas support up to 10.6 times the density of migrant birds per hectare as adjacent non-riparian areas (Stevens et al. 1977). Most of these migratory birds belong to the foliage insect (47%) or air insect (34%) foraging guilds. Coyote willow is browsed avidly by deer and to some extent by sheep, goats, and cattle, in summer and early fall. Cattle will leave the willow patches when the foliage matures and dries, whereas deer devour the current leafless stem throughout the winter. The browse rating for willow is good to fair for sheep and goats; good to poor for cattle; fair for deer; and fair to useless for horses (Sampson et al. 1981). Livestock: Riparian ecosystems offer water, shade, and food for domestic livestock. Cattle and sheep congregate in riparian areas, particularly during hot or dry periods. Overgrazing of domestic livestock in riparian areas destroys riparian ground cover, disrupts the reproductive cycle of cottonwood trees, destabilizes streambanks, and thus increases sediment loads to streams.
Status
Please consult the PLANTS Web site and your State Department of Natural Resources for this plant’s current status, such as, state noxious status and wetland indicator values.
Description
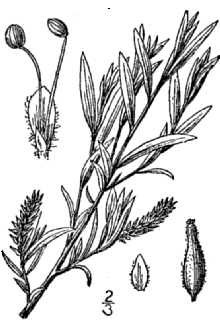
General: Willow Family (Salicaceae). Salix exigua, with its long, thin leaves, is the most distinctive of the willow species. The leaves have a very short petiole, and mature blades are 50 - 124 mm long, linear, with an acuminate leaf tip and either a serrate or entire leaf edge. Coyote willow is a shrub < 7 m tall, and spreads clonally by root-sprouting. The catkin inflorescence appears with or after the leaves in the spring, and are 22-70 mm long on leafy shoots 5-110 mm long. The flower bracts are a tawny yellow color.
Distribution
For current distribution, please consult the Plant Profile page for this species on the PLANTS Web site. Salix exigua is distributed in wetlands, along alluvial bottomlands and streamsides at elevations lower than 2700 m. Coyote willow is distributed throughout California north to Alaska, east across North America, and south to Arizona and Mexico (Hickman (1993). Mason (1957) says Salix exigua is often found at sites of former Indian habitation, and notes this was one of the common basket willows of the Indians
Establishment
Adaptation: Coyote willow dominates the riparian forests of lower terrace deposits and stabilized gravel bars. Willows are found near water; they require a bare gravel or sand substrate with adequate moisture for germination and development. Willows grow very rapidly when their roots are in contact with the permanent water table. Typically, in California, cottonwoods and willows predominate on the immediate stream banks, whereas valley oaks are spread irregularly over the natural levees farther away from stream banks. In other parts of the American west, temporal gradients occur within a location in the riparian zone. Early pioneer communities such as cottonwood/willow give way to late successional communities such as mesquite or sagebrush, often a consequence of sediment accumulation (Patten 1998). Many similarities among western riparian ecosystems exist because several dominant genera (e.g. Populus and Salix spp.) are common throughout the West, and many geomorphic and hydrologic processes that influence riparian establishment are similar. Western riparian ecosystems have been greatly altered by human activity. Riparian forests have been reduced to fragmented, discontinuous patches because of human intervention. For example, estimates are that 70 - 90 percent of the natural riparian ecosystems in the U.S. have been lost to human activities (Warner 1979). Regional losses in these ecosystems have been estimated to exceed 98% in the Sacramento Valley in California (Smith 1977) and 95% in Arizona (Warner 1979). Many factors have contributed to these resource losses, including the following: natural resource use; urbanization; alteration of stream flows through dam construction and ground-water withdrawal; modification of biotic conditions through grazing, agriculture, and introduction of non-native species; and alteration within watersheds (Patten 1998). Coyote willow roots freely from cuttings, and is an easy species to propagate. Coyote willow is a shrub 3 to 15 feet in height with multiple branches and deciduous leaves. Its architecture is resilient to disturbance such as high velocity floodwaters, sediment deposition, medium to high flooding (anoxic conditions), high winds, heavy precipitation, or pruning from beaver, deer or wildlife. Beaver browsed more than 5,000 willow cuttings to ground level in New Mexico, and all the willow resprouted (Los Lunas Plant Materials Center 1998). These cutting also survived over two months of continuous inundation. The NRCS Plant Materials Center at Los Lunas in cooperation with the U.S. Fish and Wildlife Service developed a pole planting technique for establishing willow and cottonwood (USDA, NRCS). We reprint this procedure below. • "Trial planting on well adapted sites indicate more that 80% survival of cottonwood and willow poles when dormant poles are cut and planted between November and February. • It is essential to monitor the water tables at proposed planting sites for at least one year before planting. Poles planted where the water table fluctuates widely will have lower survival rates than those planted where water table is relatively stable. If groundwater monitoring shows the water level will drop more than 3 feet during the growing season (May-October), another site should be selected. Monitoring of observation wells for at least one calendar year before planting will allow better planting depth to ensure establishment. • Salt cedar (Tamarix chinensis), Russian olive (Eleagnus angustifolia), and giant reed (Arundo donax) will need to be controlled before poles are planted. However, young cottonwoods and willows can grow successfully in quite small openings in stands of salt cedar. Study of natural stands suggest they will eventually shade out the salt cedar." Steps for Successful Pole Plantings: • Select sites as close to the area as possible to conserve genetic diversity. Try to match donor site and revegetation site in terms of soils, elevation, hydro-dynamics, permanent groundwater table, and soil salinity (which should be low). • Select willow cuttings from a local, native stand in healthy condition. Prune no more than 2/3 of plants in an area. Willow cuttings for pole plantings should generally be at least 1/2 inch in diameter or larger. Select the longest, straightest poles available. Use only two to four-year old wood. The total length of the poles needed depends upon the water table depth (see #7 below). • Measure water table fluctuations for at least 1 year, preferably longer, to determine the lowest water table depth. Take a reading at least once a month, preferably more often during the driest months of the year. • Cut poles while dormant during January and February. Remove all side branches except the top two or three. • Prepare cuttings by trimming off the top to remove the terminal bud, allowing a majority of the energy in the stem to be sent to the lateral buds for root and shot development. • Soak poles in water for at least 5 to 7 days before planting. • Dig holes to the depth of the lowest anticipated water table. Sites where the water table will be within one foot of the ground surface during the growing season are better suited for willows than cottonwoods. • The cuttings should extend several inches into the permanent water table to ensure adequate moisture for sprouting. At least 1/2 to 2/3 of the cutting should be below ground to prevent the cutting from being ripped out during high water flows. Usually, at least 2 to 3 feet should be below ground. It should also be long enough to emerge above adjacent vegetation such that it will not be shaded out. • Place cuttings in the hole the same day they are removed from the soak treatment. Set the butt as close to the lowest annual water table elevation as possible. • Electric hammer drills (Dewalt model DW530) fitted with one-inch diameter, 3-foot bits were used to plant thousands of coyote willows in New Mexico. With one drill, two people installed 500 willow per day to a 3-foot depth. A power auger or a punch bar can also be used. • Coyote willow pole cuttings were generally planted on 10 to 20 foot centers in New Mexico. Areas with a shallow water table (4-6 feet) were generally planted with a higher number of pole cuttings to enhance overall survival of the project; in this case, coyote willow was planted on 1-foot centers or even closer. Often understory species were planted under the canopy of pre-existing overstory (cottonwoods, tree willows) since they are often observed occupying this niche. • It is critical to ensure the soil is packed around the cutting to prevent air pockets. "Mudding" (filling the hole with water and then adding soil to make a mud slurry) can remove air pockets. • When necessary, install tree guards around the poles to protect from beavers, other rodents, or rabbits. Coyote willows tend to be fairly resistant to pruning from beavers, so tree guards may not be necessary. • As buds begin to swell (usually in April or May), wipe them off the lower two-thirds of the pole. This will reduce evapotranspiration water loss and stimulate root growth. • Exclude the planting area from livestock grazing for at least two to three growing seasons. There are other techniques for stabilization of banks and erosion control, called bioengineering, which utilize coyote willows. These include brush layers, brush mattresses, brush or tree revetments, brush trenches, vertical bundles, and willow wattles. Often fiberschine, erosion control fabric and hay bales are utilized to stabilize an eroding site. For further information on these techniques, refer to The Practical Streambank Bioengineering Guide by USDA, Natural Resources Conservation Service (Bentrup and Hoag 1998). Establishment From Seed: Willow seeds must be collected as soon as the capsules mature (when they turn from green to yellow) and planted immediately since they retain their viability for only a few days at room temperature. Even under the most favorable conditions, maximum storage is four to six weeks. No dormancy occurs, so germination takes place 12 to 14 hours after planting if the seeds are kept constantly moist willows are difficult to propagate in quantity by seed. Willows root so readily by either stem or root cuttings that there is little need to use other methods. Hardwood cuttings planted in early spring root promptly. For natural seed revegetation, coyote willow requires moist soil from spring over-bank flows or capillary wetting of the soil surface for establishment. A number of studies have related components of the reproductive cycle of Salix species to floodplain site conditions produced by streamflow and associated fluvial processes. In particular, components of the annual pattern of streamflow, or annual hydrograph, are associated with specific stages of Salix seedling emergence and growth. These include the following: 1) flood flows that precede Salix seed dispersal produce suitable germination sites; 2) flow recessions following a peak expose germination sites and promote seedling root elongation; and 3) base flows supply soil moisture to meet summer and winter seedling water demand (Shafroth et al. 1998; Mahoney et al. 1998). The combination of root growth and capillary fringe defines the successful recruitment band for seedling establishment, which is usually from about 0.6 to 2 m in elevation above the late summer stream stage (Mahoney et al. 1998). The rate of stream stage decline is also critical for seedling survival and should not exceed 2.5 cm per day.
Management
Traditional Resource Management: Willow is nature’s healer. Poles of willow readily sprout, and help to stabilize stream banks and provide habitat. Sweat lodges constructed of willow have been known to sprout and grow, even though the willows were subjected to very high heat. Willows were traditionally tended by pruning, to produce long straight stems. Willow is gathered only at certain times of the year, beginning in the autumn after the leaves fall. For many weavers, gathering will continue until the following spring when the sap begins to rise again. Some gatherers, once they find a good stand, will cut as much as they can. The willows in many areas have not been tended in a long time, and the stems are old, woody, and twisted. Often basket weavers will prune many willows, sometimes replanting the stems, so there will be nice straight basketry materials the following year. The Chemehuevi gather shoots, which they have burned several times, until only the living stumps of the willow, remain (Collings 1979). Straight young shoots grow from these stumps in profusion. Each twig is carefully selected. Those finally selected are at least fifteen inches long and between 1/8 and 3/16 of an inch in diameter with as little taper from end to end as possible. Before gathering, the weavers I have interviewed make offerings of thanks and pray for permission to gather (Stevens, unpublished field notes, 1998). Often tobacco or other offerings are given before beginning to gather. Basket weavers process materials with their hands and mouths. Herbicides sprayed on willows and along streams have a much higher health risk for humans when they are used for traditional materials. A Washoe basket weaver says, “Sometimes when you take the willows' skins off, they have spots from pesticides.” Another weaver says the plants then grow deformed; the shoots don't grow straight and the willows are bumpy and wormy inside (Fulkerson 1995). Howe and Knopf (1991) conclude that to ensure the survival of willows and cottonwoods in riparian communities, resource managers need to implement strategies to control the spread of exotic species. Livestock grazing has widely been identified as a leading factor causing or contributing to degradation of riparian habitats in the western United States (U.S. General Accounting Office 1988; Chaney et al. 1990, Fleischner 1994, Ohmart 1996). Livestock grazing can alter vegetative structure and composition of riparian habitat. Overgrazing, especially by livestock and big game, frequently changes plant species composition and growth form, density of stands, vigor, seed production of plants, and insect production. Livestock grazing can cause the replacement of bird and mammal species requiring the vertical vegetation structure of riparian habitat to species, which are ubiquitous in their habitat preferences. Slovlin (1984) recommended a 5-year rest from cattle grazing to re-establish healthy stands of riparian vegetation, such as cottonwood and willows. Siekert et al. (1985) reported that spring grazing showed no significant changes in channel morphology, whereas summer and fall grazing did. However, even with limited seasonal grazing, all tree seedlings would be eliminated. Marlow and Pogacnik (1985) recommended fencing riparian habitat, rest-rotation, light grazing (<20% forage removal), and grazing after streambanks have dried to 10% moisture. Cultivars, Improved and Selected Materials (and area of origin) Containerized coyote willow saplings are available from most nurseries in the areas where adapted. We recommend using plants from the same region, elevation, climate, soil type, moisture or hydrologic regime as you are replanting. Coyote willow poles, suitable for transplanting, are available from the NRCS Plant Materials Center at Los Lunas, New Mexico and Tucson, Arizona. The Plant Materials Centers vegetatively propagate these poles from parent stock. Each center maintains parent stock of several ecotypes collected from the center's NRCS service area. These ecotype collections vary in the amount of genetic diversity within ecotypes. These centers can supply poles to NRCS Field and State Offices, and other public agencies. Contact your local Natural Resources
Conservation
Service (formerly Soil Conservation Service) office for more information. Look in the phone book under ”United States Government.” The Natural Resources Conservation Service will be listed under the subheading “Department of Agriculture.”
References
Auble, G.T. & M.L. Scott 1998. Fluvial disturbance patches and cottonwood recruitment along the upper Missouri River, Montana. Wetland 18(4): 546-556. Baird, K. 1989. High quality restoration of riparian ecosystems. Restoration and Management Notes 7(2):60-64. Beier, P. & R.H. Barret 1987. Beaver habitat use and impact in the Truckee River basin, California USA. J. Wildlife Management 51: 794-799. Bentrup, G. & J.C. Hoag 1998. The practical streambank bioengineering guide. User's guide for natural streambank stabilization techniques in the arid and semi-arid Great Basin and Intermountain West. USDA, NRCS, Plant Materials Center, Aberdeen, Idaho. Brode, J. & R.B. Bury 1984. The importance of riparian systems to amphibians and reptiles. Pages 30 - 35 IN: R.E. Warner and K. Hendrix, eds. California riparian systems; ecology, conservation, and productive management. University of California Press, Berkeley, California. Brotherson, J.D., S.R. Rushford, W.E. Evenson, & C. Morden 1983. Population dynamics and age relationships of eight trees in Navajo National Monument, Arizona. J. Range Management 36: 250-256. Brunsfeld, S.J. & F.D. Johnson 1985. Field guide to the willows of east-central Idaho. Forest,
Wildlife
and Range Experiment Station. University of Idaho Bull. #39. Bull, E.L. & J.N. Slovlen 1982. Relationships between avifauna and streamside vegetation. Trans. North. Am. Wildl. Nat. Resour. Conf. 47: 496-506. Cemments, C. 1991. Beavers and riparian systems. Rangelands 13:277-279. Chaney, E., W. Elmore, & W.S. Platts 1990. Livestock grazing on western riparian areas. U.S. Environmental Protection Agency, Region 8, Denver, Colorado. Collings, J.L. 1979. Profile of a Chemehuevi basket weaver. American Indian Art Magazine (Autumn). pp 3-11. Conard, S.G., R.L. MacDonald & R.F. Holland 1977. Riparian vegetation and flora of the Sacramento Valley. Pages 47-56, IN: Anne Sand (ed.), Riparian Forests in California. Their Ecology and Conservation. Crouch, G.L. 1979. Long-term changes in cottonwoods on a grazed and ungrazed Plains bottomland in Northeastern Colorado. USDA, Forest Service Research Note RM 370: 1-4. Ditterberner, P.L. & M.R. Olson 1983. The plant information network (PIN) data base Colorado, Montana, North Dakota, Utah, and Wyoming. U.S. Fish and Wildlife Service FWS/OBS-83/36. Dunmire, W.W. & G.D. Tierney 1997. Wild plants and native peoples of the Four Corners. Museum of New Mexico Press, Santa Fe, New Mexico. 312 pp. Dunmire, W.W. & G.D. Tierney 1995. Wild plants of the Pueblo province. Exploring ancient and enduring use. Museum of New Mexico Press, Santa Fe, New Mexico. 290 pp. Ellis, L.M. 1994. Bird use of salt cedar and cottonwoods on a grazed and ungrazed plains bottomland in Northeastern Colorado. USDA, Forest Service Research Note RM-370:1-4. Farley, G.H., L.M. Ellis, J.N. Stuart, & N.J. Scott, Jr. 1994. Avian species richness in different-aged stands of riparian forest along the middle Rio Grande, New Mexico. Conservation Biology 8:1098-1108. Fenner, P.W., W.W. Brady, & D.R. Patton 1984. Observations on seed and seedlings of Fremont's cottonwood. Desert Plants 6:55-58. Fleishner, T.L. 1994. Ecological costs of livestock grazing in western North America. Conservation Biology 8:629-644. Fowler, C.S. 1992. In the shadow of Fox Peak. An ethnography of the cattail-eater Northern Paiute people of Stillwater Marsh. Cultural Resource Series Number 5. U.S. Department of the Interior. Fish and Wildlife Service, Region 1. Stillwater National Wildlife Refuge. 264 pp. Fulkerson, M.L. 1995. Weavers of tradition and beauty. Basketmakers of the Great Basin. University of Nevada Press. 138 pp. Gaines, D. 1977. The valley riparian forests of California: Their importance to bird populations. Pages 57-86, IN: Anne Sand (ed.), Riparian Forests in California. Their Ecology and Conservation. Glinski, R.L. 1977. Regeneration and distribution of sycamore and cottonwood trees along Sonoita Creek, Santa Cruz County, Arizona. USDA, Forest Service Gen. Tech. Rep. RM-43:116-123. Grenfell, W.E., Jr. 1988. Valley foothill riparian. Pages 86-87, IN: Kenneth A. Mayer and William F. Laudenslayer, Jr. A guide to wildlife habitats of California. USDA - Pacific SW Forest and
Range
Experiment Station, California Dept. of Fish and Game, PG and E, and USDA Forest Service Region 5. Grime, J.P. 1978. Interpretation of small-scale patterns in the distribution of plant species in space and time. Pages 101-104, IN: A.J.H. Freysen and J.W. Woldendorp (eds.) Structure and Functioning of Plant Populations. Elsevier. North-Holland, Amsterdam, New York. Grime, J.P. and R. Hunt 1975. Relative growth rate: its range and adaptive significance in a local flora. J. Ecology 63: 393-422. Hartmann, H.T., D.E. Kesler, & F.T. Davies, Jr. 1990. Plant propagation principles and practices. Prentice Hall, Englewood Cliffs, New Jersey. 647 pp. Hickman, J.C. (ed.) 1993. The Jepson manual. Higher plants of California. University of California Press. 1400 pp. Hoag, J.C. 1992. Use of willow and cottonwood cuttings for vegetation shorelines and riparian areas. USDA, NRCS, Riparian/Wetland Project Information Series #3, Plant Materials Center, Aberdeen, Idaho. Hoag, J.C. 1993a. Selection and acquisition of woody plant species and materials for riparian corridors and shorelines. Riparian/Wetland Project Information Series #2. USDA, NRCS, Plant Materials Center, Aberdeen, Idaho. Hoag, J.C. 1993b. How to plant willows and cottonwood for riparian rehabilitation. Idaho Plant Materials Technical Note #23. USDA NRCS, Boise, Idaho. Howe, W.H. & R.L. Knopf 1991. On the imminent decline of Rio Grande cottonwoods in central New Mexico. The Southwestern Naturalist 36:28-224. Johnson, R.R. & C.W. Lowe 1985. On the development of riparian ecology. Pages 112-116 IN: R.R. Johnson, C.D. Ziebell, D.R. Patten, P.F. Ffolliot, and R.H. Hamre (tech. coord.) Riparian ecosystems and their management: Reconciling conflicting uses. General Technical Report RM-120. USDA, Forest Service, Fort Collins, Colorado. Kindscher, K. 1992. Medicinal wild plants of the prairie. An ethnobotanical guide. University Press of Kansas. 340 pp. Knopf, F.I. and F.B. Samson 1994. Scale perspectives on avian diversity in western riparian ecosystems. Conservation Biology 8(3):669-676. Laymon, S.A. 1984. Riparian bird community structure and dynamics: Dog Island, Red Bluff, California. Pages 587-597 IN: R.E. Warner and K. Hendrix, eds. California riparian systems; ecology, conservation, and productive management. Univ. of California Press, Berkeley, California. Mahoney, J.M. & S.B. Rood 1998. Streamflow requirements for cottonwood seedling recruitment - an integrative model. Wetlands 18(4): 634-645. Marlow, C.B. & T.M. Pogacnik 1985. Time of grazing and cattle-induced damage to streambanks. Pages 279-284 IN: Johnson, R.R., C.D. Ziebell, D.R. Patton, P.F. Ffolliott, and R.H. Hamre (Tech. Coords). Riparian Ecosystems and Their Management: Reconciling Conflicting Uses. Proc. First North Am. Riparian Conf. USDA, For. Serv. Gen. Tech. Rep. RM-120. 523 pp. Martin, A.C., H.S. Zim, & A.L. Nelson 1951. American wildlife and plants: A guide to wildlife food habits. Dover Publications, Inc., New York, New York. 500 pp. McGinley, M.A. & T.G. Whitham 1985. Central place foraging by beavers (Castor canadensis): A test of foraging predictions and the impact of selective feeding on the growth form of cottonwoods (Populus fremontii). Oecologia 66: 558-562. Michny, F.J., D. Boos & F. Wernette 1974. Riparian habitats and avian densities along the Sacramento River, California. State of California Dept. of Fish and Game. 23 pages. Moore, M. 1979. Medicinal plants of the Mountain West. Museum of New Mexico Press. 200 pp. Ohmart, R.D. 1996. Historical and present impacts of livestock grazing on fish and wildlife resources in western riparian habitats. Pages 245-279 IN: P.R. Karausman (ed.) Rangeland Wildlife. Society for Range Management, Denver, Colorado, USA. Ohmart, R.D. & B.W. Anderson 1986. Riparian habitat. In inventory and monitoring of wildlife habitat. Bureau of Land Management. pp 169-199. Patten, D.T. 1998. Riparian ecosystems of semi-arid North America: diversity and human impacts. Wetland 18(4): 498-512. Platts, W. et al. 1987. Methods for evaluating riparian habitat with applications to management. USDA, Forest Service, Intermountain Research Station, General Technical Report INT-221. Pope, D.P., J.H. Brock, & R.A. Backhaus 1990. Vegetative propagation of key Southwestern woody riparian species. Desert Plants 10: 91-95. Reichenbacher, F.W. 1984. Ecology and evolution of Southwestern riparian plant communities. Desert Plants 6:15-22. Roberts, W.G, J.G. Howe & J. Major 1977. A survey of riparian forest flora and fauna in California. Pages 3-20 IN: Anne Sand (ed.). Riparian Forests in California. Their Ecology and Conservation. Robichaux, R. 1977. Geologic history of the riparian forests of California. Pages 21-34 IN: Anne Sand (ed.). Riparian Forests in California. Their Ecology and Conservation. Rucks, M.G. 1984. Composition and trend of riparian vegetation on five perennial streams in southeastern Arizona. pp 97-107 IN: R.E. Warner and K.M. Hendrix (eds.). California Riparian Systems: Ecology, Conservation, and Productive Management. University of California Press, Berkeley, California. St. John, T.V. 1987. Mineral acquisition in native plants. Pages 529-536 IN: Elias, Thomas S. (ed.) Conservation and Management of Rare and Endangered Plants. California Native Plant Society, Sacramento, California. St. John, T.V. 1988. Soil disturbance and the mineral nutrient of native plants. Pages 34-39 IN: Rieger J.P. and B.K. Williams (eds.) Proceedings of the Second Native Plant Revegetation Symposium. San Diego, California. Sampson, A.W. & B.S. Jespersen 1981. California range brushlands and browse plants. Division of Agricultural Sciences. University of California. Schulz, T.T. & W.C. Leininger 1991. Nongame wildlife communities in grazed and ungrazed montane riparian sites. Great Basin Naturalist 51(3):286-292. Schulz, T.T. & W.C. Leininger 1990. Differences in riparian vegetation structure between grazed areas and exclosures. Journal of Range Management 43(4):295-299. Shafroth, P.B., G.T. Auble, J.C. Stromberg, & D.T. Patten 1998. Establishment of woody riparian vegetation in relation to annual patterns of streamflow, Bill Williams River, Arizona. Wetlands (18)4:577-590. Shanefield, A.N. 1984. Alder, cottonwood, and sycamore distribution and regeneration along the Nacimiento River, California. pp 196-201 IN: R.E. Warner and K.M. Hendrix (eds.). California Riparian Systems: Ecology, Conservation, and Productive Management. University of California Press, Berkeley, California. Siekert, R.E., Q.D. Skinner, M.A. Smith, J.L. Dodd, & J.D. Rodgers 1985. Channel response of an ephemeral stream in Wyoming to selected grazing treatments. Pages 276-278 IN: Johnson, R.R., C.D. Ziebell, D.R. Patton, P.F. Ffolliott, and R.H. Hamre (Tech. coords). Riparian Ecosystems and Their Management: Reconciling Conflicting Uses. Proc. First North Am. Riparian Conf. USDA, For. Serv. Gen. Tech. Rep. RM-120. 523 pp. Slovlin, J.M. 1984. Impact of grazing on wetlands and riparian habitat: a review of our knowledge. Pages 1001-1104 IN: Developing strategies for rangeland management. Nat. Res. Counc. Natl. Acad. Sci. Westview Press, Boulder, Colorado. Smith, F. 1977. A short review of the status of riparian forests in California. Pages 1-2 IN: Anne Sand (ed.). Riparian Forests in California. Their Ecology and Conservation. Stevens, L.E., B.T. Brown, J.M. Simpson, & R.R. Johnson 1977. The importance of riparian habitat to migrating birds. Pages 156-164 IN: Johnson, R.R. and D.A. Jones (tech. coords.). Importance, preservation and management of riparian habitat: a symposium. USDA, Forest Service Gen. Tech. Report RM-43. Rocky Mountain Forest and Range Experiment Station, Fort Collins, Colorado. 217 pp. Strike, S.S. 1994. Ethnobotany of the California Indians. Volume 2. Aboriginal Uses of California's Indigenous Plants. Koelz Scientific Books USA/Germany. 210 pp. Stromberg, J.C. 1993. Fremont cottonwood-goodding willow riparian forest: a review of their ecology, threats, and recovery potential. J. Arizona-Nevada Acad. Sci. 27:97-110. Stromberg, J.C. 1998. Functional equivalency of saltcedar (Tamarix chinensis) and Fremont cottonwood (Populus fremontii) along a free-flowing river. Wetlands 18(4):675-686. Thompson, K. 1977. Riparian forests of the Sacramento Valley, California. Pages 35-38 IN: Anne Sand (ed.). Riparian Forests in California. Their Ecology and Conservation. Tilford, G.L. 1997. Edible and medicinal plants of the West. Mountain Press Publishing Company, Missoula, Montana. Trapp, G.R., G.L. Linck & E.D. Whisler 1984. The status of ecological research on the mammal fauna of California's central valley riparian communities. Pages 942-949 IN: R.E. Warner and K. Hendrix, eds. California riparian systems; ecology, conservation, and productive management. University of California Press, Berkeley, California. USDA, Natural Resources Conservation Service 1992. Soil bioengineering for upland slope protection and erosion protection. USDA, NRCS, Engineering Field Handbook. Chapter 18. USDA, Natural Resources Conservation Service 1998. 1998 annual interagency riparian report. Plant Materials Center, Los Lunas, New Mexico. USDA, NRCS 1999. The PLANTS database. National Plant Data Center, Baton Rouge, Louisiana. <http://plants.usda.gov> Version: 990405. U. S. General Accounting Office 1988. Public rangelands: some riparian areas restored but widespread improvement will be slow. Washington, DC, USA. GAO/RCED-88-01. Warner, R.E. and K.M. Hendrix 1979. California riparian systems: Ecology, conservation, and productive management. University of California Press, Berkeley, California. Wheat, M.M. 1967. Survival arts of the primitive Paiutes. University of Nevada Press, Reno, Nevada. 117 pp. Williams, D.F. & K.S. Kilburn 1984. Sensitive, threatened, and endangered mammals of riparian and other wetland communities in California. Pages 950-956 IN: Warner, R.E., and K.E. Hendrix (eds.), California riparian systems: ecology, conservation, and productive management. University of California Press, Berkeley, California. 1035 pp.
Fact Sheet
Alternate Names
narrowleaf willow
Uses
Erosion control: Sandbar willow is used for streambank and lake shore stabilization and riparian area development or restoration. It is recommended for deep wet lowland, overflow areas, wet meadow sites, streambanks, lake shores, and other areas with a high water table. Wildlife: This plant is provides wood and shelter for many game birds and forage for deer.
Status
Please consult the PLANTS Web site and your State Department of Natural Resources for this plant’s current status (e, Use soil moisture sensors to measure the soil moisture of Salix sessilifolia Nutt. ssp. hindsiana (Benth.) Andersson.,g, threatened or endangered species, state noxious status, and wetland indicator values),
Weediness
This plant may become weedy or invasive in some regions or habitats and may displace desirable vegetation if not properly managed. Please consult with your local NRCS Field Office, Cooperative Extension Service office, or state natural resource or agriculture department regarding its status and use. Weed information is also available from the PLANTS Web site at plants.usda.gov.
Description
Salix exigua Nutt., sandbar willow, is a common native suckering shrub 3 to 20 feet high found throughout the Northern Great Plains and the Northeast US. It quickly forms thickets on sand or gravel deposits along streams, roadside ditches, sloughs, and other places frequent to flooding. Branchlets are reddish brown, smooth or nearly so. Leaves are 2 to 5 inches long, narrowly lance-shaped, and pointed at both ends, with margins that have shallow, widely spaced teeth; they are green and smooth on both surfaces or sometimes silvery-silky. Leafstalks are very short and stipules, if present, are very small. This shrub is dioecious, so male and female flowers are produced by separate plants. Sandbar willow leaves are very narrow with serrated leaf edges. The leaf edges of purpleosier willow are not serrated, and the leaf width is greater. Also, purpleosier willow does not form thickets. Robert H. Mohlenc\brock USDA NRCS 1989 Midwestern Wetland Flora @ USDA NRCS PLANTS Note: sandbar willow is an aggressive spreader and this should be considered when selecting materials for a given site. It can spread off of the streambank to other sites under favorable circumstances.
Adaptation and Distribution
Distribution
Distribution
Sandbar willow is adapted to sandy soils in stream, river, and shoreline sites, it is not well adapted to other sites. Sandbar willow is distributed primarily throughout the West. For a current distribution map, please consult the Plant Profile page for this species on the PLANTS Website.
Establishment
Planting 1-0 rooted cuttings or unrooted cuttings are both effective planting methods. The un-rooted cuttings should be used where moisture conditions are good. On droughty sites, the rooted cuttings are preferred. Plant rooted cuttings using techniques that are common to bare root seedlings. Un-rooted cuttings should be at least 12 inches long, with the lower 10 inches buried vertically in the sand. Plant spacing of 2x2 to 4x4 work well. Sandbar willow is also planted in soil bioengineering systems. It should be planted in mixtures with other species such as ‘Streamco’ and ‘Bankers’ willows and ‘Ruby’ dogwood for live fascines, brush layers and brush mattress. Under-seeding with a cool season grass mixture is recommended.
Management
Once sandbar willow is planted, it requires little care. Blowouts along the stream should be addressed when they occur and repaired.
Plant Traits
Growth Requirements
| Temperature, Minimum (°F) | -38 |
|---|---|
| Adapted to Coarse Textured Soils | Yes |
| Adapted to Fine Textured Soils | No |
| Adapted to Medium Textured Soils | Yes |
| Anaerobic Tolerance | High |
| CaCO3 Tolerance | High |
| Cold Stratification Required | Yes |
| Drought Tolerance | Medium |
| Fertility Requirement | Low |
| Fire Tolerance | High |
| Frost Free Days, Minimum | 120 |
| Hedge Tolerance | Medium |
| Moisture Use | High |
| pH, Maximum | 8.5 |
| pH, Minimum | 6.0 |
| Planting Density per Acre, Maxim | 1200 |
| Planting Density per Acre, Minim | 700 |
| Precipitation, Maximum | 30 |
| Precipitation, Minimum | 20 |
| Root Depth, Minimum (inches) | 20 |
| Salinity Tolerance | Low |
| Shade Tolerance | Intermediate |
Morphology/Physiology
| Bloat | None |
|---|---|
| Toxicity | None |
| Resprout Ability | Yes |
| Shape and Orientation | Erect |
| Active Growth Period | Spring and Summer |
| C:N Ratio | Medium |
| Coppice Potential | No |
| Fall Conspicuous | No |
| Fire Resistant | No |
| Flower Color | Yellow |
| Flower Conspicuous | No |
| Foliage Color | Green |
| Foliage Porosity Summer | Dense |
| Foliage Porosity Winter | Porous |
| Foliage Texture | Medium |
| Fruit/Seed Conspicuous | No |
| Nitrogen Fixation | None |
| Low Growing Grass | No |
| Lifespan | Moderate |
| Leaf Retention | No |
| Known Allelopath | No |
| Height, Mature (feet) | 10.0 |
| Height at 20 Years, Maximum (fee | 10 |
| Growth Rate | Rapid |
| Growth Form | Rhizomatous |
| Fruit/Seed Color | White |
Reproduction
| Vegetative Spread Rate | Moderate |
|---|---|
| Small Grain | No |
| Seedling Vigor | Medium |
| Seed Spread Rate | Moderate |
| Fruit/Seed Period End | Spring |
| Seed per Pound | 1000000 |
| Propagated by Tubers | No |
| Propagated by Sprigs | No |
| Propagated by Sod | No |
| Propagated by Seed | No |
| Propagated by Corm | No |
| Propagated by Container | Yes |
| Propagated by Bulb | No |
| Propagated by Bare Root | Yes |
| Fruit/Seed Persistence | No |
| Fruit/Seed Period Begin | Summer |
| Fruit/Seed Abundance | High |
| Commercial Availability | Routinely Available |
| Bloom Period | Early Spring |
| Propagated by Cuttings | Yes |
Suitability/Use
| Veneer Product | No |
|---|---|
| Pulpwood Product | No |
| Protein Potential | Low |
| Post Product | No |
| Palatable Human | No |
| Palatable Graze Animal | Low |
| Palatable Browse Animal | Low |
| Nursery Stock Product | Yes |
| Naval Store Product | No |
| Lumber Product | No |
| Fodder Product | No |
| Christmas Tree Product | No |
| Berry/Nut/Seed Product | No |