Salix nigra Marshall var. amygdaloides (Andersson) Andersson
Scientific Name: Salix nigra Marshall var. amygdaloides (Andersson) Andersson

| General Information | |
|---|---|
| Usda Symbol | SANIA |
| Group | Dicot |
| Life Cycle | Perennial |
| Growth Habits | ShrubTree, |
| Native Locations | SANIA |
Plant Guide
Alternative Names
Wright willow, almond willow, willow , Use soil moisture sensors to measure the soil moisture of Salix nigra Marshall var. amygdaloides (Andersson) Andersson.
Uses
Willows were used for making dye, furniture, mats, baskets, drums, stirrups, tipi pegs and pins, fox and fish traps, hunting lodge poles, and meat-drying racks (Kindscher 1992). Willows were and still are used for baskets throughout their range. The Paiute, Ute, Shoshone, Hopi, Havasupai, Mandan, Cheyenne, Arapaho, Kiowa, and others use Salix lucida for basketweaving (James 1972, Mason 1988). Kelly Kindscher(1992) wrote in Medicinal Wild Plants of the Prairie: "The Blackfeet made a tea from the fresh root of Salix species to treat internal hemorrhage, throat constrictions, swollen neck glands, and bloodshot or irritated eyes. The twigs were also gathered and preserved. Steeped in boiling water, they were made into a tea to cure fever or alleviate pain." Salix species were used as chew sticks to clean teeth by many other Indian tribes, including the Choctaw, Delaware, and Cheyenne. The peachleaf willow was favored by the Osage, Delaware, and Cherokee for this purpose (Elvin-Lewis 1979). The Kiowa made a tea of willow leaves, which they rubbed on the body to cure pneumonia and relieve rheumatic aches. They also chewed the bark to relieve toothaches (Vestal and Shultes 1939). The Comanche burned the stems of the willow and used the ashes to treat sore eyes (Carlson and Jones 1939). To restore themselves both physically and mentally, the Dakota drank a willow-bark tea (Andros 1883). The Ojibwe used peachleaf willow bark externally to treat skin rashes. Robert Mollenbrock USDA, NRCS, Wetland Science Institute @ PLANTS Aspirin is the pharmaceutical equivalent of willow bark tea, which is an effective remedy for headache, fever or sore throat. More than 2,400 years ago, the Greeks learned to use extracts of several native willow species to treat pain, gout, and other illnesses. In more recent times, in 1839, salicylic acid was isolated from wild plants and manufactured synthetically. Early salicylic acid-based products had unpleasant side effects. Sixty years later, the Bayer Company developed a derivative of salicylic acid, called it aspirin, and the rest is history. Tea made from willow leaves will cure laryngitis. Willow reduces inflammation of joints and membranes (Moore 1979). When used as an analgesic, willow treats urethra and bladder irritation, infected wounds, and eczema. Willow is used as an over-all treatment of many diseases, including hay fever, diarrhea, prostatitis, satyriasis, and as a relief of ovarian pain. A poultice is made for treating gangrene and skin ulcers. Young willow shoots can be stripped of their bark and eaten. The young leaves may be eaten in case of emergency. The inner bark can be eaten raw, prepared like spaghetti, or made into flour. Riparian: Peachleaf willow is an overstory dominant species in many riparian ecosystems throughout the American west and midwest. Riparian ecosystem functions provided by willows include the following: 1) Riparian vegetation traps sediments and nutrients from surface runoff and prevents them from entering the aquatic system; 2) the dense matrix of roots in the riparian zone can serve as an effective filter of shallow groundwater; 3) water quality is improved through filtration and the trapping of sediment, nutrients (particularly nitrogen dissolved in groundwater), and pollutants; and 4) riparian areas act as a sponge by absorbing floodwaters. The water is then slowly released over a period of time, which minimizes flood damage and sustains higher base flows during late summer. Wildlife: Structurally complex riparian vegetation communities provide many different habitats and support a diverse array of animal species. The multiple layers of vegetation provide multiple niches for many species of insects and wildlife canopies of plants growing on streambank provide shade, cooling stream water, while roots stabilize and create overhanging banks, providing habitat for fish and other aquatic organisms. Rabbits and many ungulates (including deer, moose, and elk) browse on willow twigs, foliage, and bark (Martin 1951). Beaver love willow branches. Several species of birds eat willow buds and young twigs. Riparian forests support a high diversity of breeding birds (Miller 1951). The percentage of breeding individuals, which are migratory, is very high in the cottonwood-willow habitat. Moister conditions in the cottonwood-willow forest may promote lusher plant growth, higher invertebrate populations and, therefore, more available food for flycatchers, warblers, and other migratory, insectivorous birds. Riparian areas support up to 10.6 times the density of migrant birds per hectare as adjacent non-riparian areas (Stevens et al. 1977). Most of these migratory birds belong to the foliage insect (47%) or air insect (34%) foraging guilds.
Status
Please consult the PLANTS Web site and your State Department of Natural Resources for this plant’s current status, such as, state noxious status, and wetland indicator values.
Description
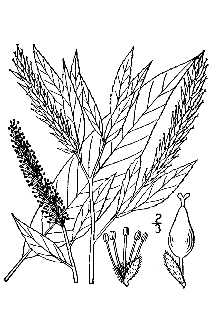
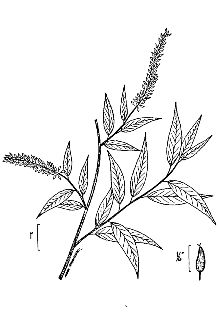
Willow Family (Salicaceae). Peachleaf willow (Salix amygdaloides) is a small to medium sized tree with one to several trunks up to 12 m tall (40 feet) (McGregor et al. 1986, Stephens 1973). The twigs are gray to light yellow, shiny, and flexible. The leaves look like peach leaves; they are yellowish green above, pale to white-glaucous beneath, glabrous, lance-shaped, 3-8 cm (1.2-3") long and finely serrate. The petioles are glandless. Catkins emerge with the leaves; pistillate (female) catkins are 3-8 cm long, on leaf branchlets 1-4 cm long. Bracts are deciduous, pale yellow, and villous on the inside. The fruits are ovoid capsules 3-5 mm long, glabrous, uncrowded on the axis giving the catkin a loose, open appearance. When ripe, the capsules open to release tiny wind-born seeds with silky hairs at their base. Peachleaf willow flowers in May and fruits in June.
Distribution
Peachleaf willow grows in riparian areas such as the banks of streams and ponds, low woods, roadside gullies, and prairie sloughs. It ranges from Quebec, west across southern Canada to British Columbia, south to Oregon, Utah, and Arizona, east to Texas, and northeast to Kentucky and Vermont. For current distribution, please consult the Plant Profile page for this species on the PLANTS Web site.
Establishment
Willows root freely from cuttings, and are easy to propagate. Willows are difficult to propagate in quantity by seed. The NRCS, Plant Materials Center, Los Lunas, New Mexico, in cooperation with the U.S. Fish and Wildlife Service, developed a pole planting technique for establishing willow (Hoag 1993a). We reprint this procedure below. "Trial planting on well adapted sites indicate more that 80% survival of cottonwood and willow poles when dormant poles are cut and planted between November and February. It is essential to monitor the water tables at proposed planting sites for at least one year before planting. Poles planted where the water table fluctuates widely will have lower survival rates than those planted where water table is relatively stable. If groundwater monitoring shows the water level will drop more than 3 feet during the growing season (May-October), another site should be selected. Monitoring of observation wells for at least one calendar year before planting will allow better planting depth to ensure establishment." Steps for Successful Pole Plantings • Select collection sites as close to the area as possible to conserve genetic diversity. Try to match donor site and revegetation site in terms of soils, elevation, hydro-dynamics, permanent groundwater table, and soil salinity (which should be low). • Select willow cuttings from a local, native stand in healthy condition. Prune no more than 2/3 of plants in an area. Willow cuttings for pole plantings should generally be at least 1/2 inch in diameter or larger. Select the longest, straightest poles available. Use only two to four-year old wood. The total length of the poles needed depends upon the water table depth. • Measure water table fluctuations in the planting area for at least 1 year, preferably longer, to determine the lowest water table depth. Take a reading at least once a month, preferably more often during the driest months of the year. Cut poles while dormant. Remove all side branches except the top two or three. • Prepare cuttings by trimming off the top to remove the terminal bud, allowing a majority of the energy in the stem to be sent to the lateral buds for root and shot development. • Soak poles in water for at least 5 to 7 days before planting. • Dig holes to the depth of the lowest anticipated water table. Sites where the water table will be within one foot of the ground surface during the growing season are better suited for willows than cottonwoods. • The cuttings should extend several inches into the permanent water table to ensure adequate moisture for sprouting. At least 1/2 to 2/3's of the cutting should be below ground to prevent the cutting from being ripped out during high flows. Usually, at least 2 to 3 feet should be below ground. It should also be long enough to emerge above adjacent vegetation such that it will not be shaded out. • Place the cuttings in the holes the same day they were removed from the soak treatment. Set the butt as close to the lowest annual water table elevation as possible. • Electric hammer drills (Dewalt model DW530) fitted with one-inch diameter, 3-foot bits were used to plant thousands of willows in New Mexico. With one drill, two people installed 500 willow cuttings per day to a 3-foot depth. A power auger or a punch bar can also be used. • Willow pole cuttings were generally planted on 10 to 20 foot centers in New Mexico. Areas with a shallow water table (4-6 feet) were generally planted with a higher number of pole cuttings to enhance overall survival. Often understory species were planted under the canopy of pre-existing overstory (cottonwoods, tree willows), since they are often observed occupying this niche. • It is critical to ensure that the soil is packed around the cutting to prevent air pockets. "Mudding" (filling the hole with water and then adding soil to make mud slurry) can remove air pockets. • When necessary, install tree guards around the poles to protect from beavers, other rodents, or rabbits. Willows tend to be fairly resistant to pruning from beavers, so tree guards may not be necessary. • As buds begin to swell (usually in April or May), remove them from the lower two-thirds of the pole. This will reduce evapo-transpiration water loss and stimulate root growth. • Exclude the planting area from livestock grazing for at least two to three growing seasons.
Seed Collections
• Willow seeds must be collected as soon as the capsules mature (when they turn from green to yellow or tan). • Plant seeds immediately, since they retain their viability for only a few days at room temperature. Even under the most favorable conditions, maximum storage is four to six weeks. • Germination takes place 12 to 14 hours after planting. Keep soil moist while seedlings germinate and grow. • When seeding outdoors, willows require moist soil from spring over-bank flows, capillary wetting of the soil surface, or irrigation for establishment.
Management
Traditional resource management of willow includes the following: • Willows were traditionally tended by pruning or burning to produce long straight stems. • Willow is gathered only at certain times of the year, beginning in the autumn after the leaves fall. For many weavers, gathering will continue until the following spring when the sap begins to rise again. • Often, basketweavers will prune many willows, sometimes replanting the stems, so there will be nice straight basketry materials the following year. • Before gathering, the weavers make offerings of thanks and pray for permission to gather. Often tobacco or other offerings are given before beginning to gather. • Basket weavers process materials with their hands and mouths. Herbicides sprayed on willows and along streams have a much higher health risk for humans when they are processed and used for traditional materials. Howe and Knopf (1991) conclude that to ensure the survival of willows and cottonwoods in riparian communities, resource managers need to implement strategies to control the spread of exotic species. Livestock grazing has widely been identified as a leading factor causing or contributing to degradation of riparian habitats in the western United States (Chaney et al. 1990, Fleischner 1994, Ohmart 1996). Livestock grazing can alter vegetative structure and composition of riparian habitat. Overgrazing, especially by livestock and big game, frequently changes plant species composition and growth form, density of stands, vigor, seed production of plants, and insect production. Livestock grazing can cause the replacement of bird and mammal species requiring the vertical vegetation structure of riparian habitat to species, which are ubiquitous in their habitat preferences. Previous heavy cattle grazing changed the bird and small mammal community composition in riparian areas through reduction of shrub and herbaceous cover. Slovlin (1984) recommended a 5-year rest from cattle grazing to re-establish healthy stands of riparian vegetation such as cottonwood and willows. Siekert et al. (1985) reported that spring grazing showed no significant changes in channel morphology, whereas summer and fall grazing did. However, even with limited seasonal grazing, all tree seedlings would be eliminated. Marlow and Pogacnik (1985) recommended fencing riparian habitat, rest-rotation, light grazing (<20% forage removal), and grazing after streambanks have dried to 10% moisture. Cultivars, Improved and Selected Materials (and area of origin) Containerized peachleaf willow saplings are available from most nurseries in the areas where they grow. We recommend using plants from the same region, elevation, climate, soil type, moisture, or hydrologic regime as you are replanting. Contact your local Natural Resources
Conservation
Service (formerly Soil Conservation Service) office for more information. Look in the phone book under ”United States Government.” The Natural Resources Conservation Service will be listed under the subheading “Department of Agriculture.”
References
Andros, F. 1883. The medicine and surgery of the Winnebago and Dakota Indians. American Medical Association Journal 1: 116-118. Baird, K. 1989. High quality restoration of riparian ecosystems. Restoration and Management Notes 7(2):60-64. Bentrup, G. & J. C. Hoag 1998. The practical streambank bioengineering guide. User's guide for natural streambank stabilization techniques in the arid and semi-arid Great Basin and Intermountain West. USDA, NRCS, Plant Materials Center, Aberdeen, Idaho. Carlson, G.G. & V.H. Jones 1939. Some notes on use of plants by the Comanche Indians. Michigan Academy of Science, Arts, and Letters 25: 517-543. Ellis, L.M. 1994. Bird use of salt cedar and cottonwoods on a grazed and ungrazed plains bottomland in Northeastern Colorado. USDA, FS, Research Note RM-370:1-4. Elvin-Lewis, M. 1979. Empirical rationale for teeth cleaning plant selection. Medical Anthropology (Fall) 1979:431-456. Farley, G.H., L.M. Ellis, J.N. Stuart, & N.J. Scott, Jr. 1994. Avian species richness in different-aged stands of riparian forest along the middle Rio Grande, New Mexico. Conservation Biology 8:1098-1108. Fleishner, T.L. 1994. Ecological costs of livestock grazing in western North America. Conservation Biology 8:629-644. Hartmann, H.T., D.E. Kesler, & F.T. Davies, Jr. 1990. Plant propagation principles and practices. Prentice Hall, Englewood Cliffs, New Jersey. 647 pp. Hoag, J.C. 1992. Use of willow and cottonwood cuttings for vegetation shorelines and riparian areas. USDA NRCS Riparian/Wetland Project Information Series #3, Plant Materials Center, Aberdeen, Idaho. Hoag, J.C. 1993a. Selection and acquisition of woody plant species and materials for riparian corridors and shorelines. Riparian/Wetland Project Information Series #2, USDA, NRCS, Plant Materials Center, Aberdeen, Idaho. Hoag, J.C. 1993b. How to plant willows and cottonwood for riparian rehabilitation. Technical Note #23, USDA, NRCS, Idaho Plant Materials, Boise, Idaho. James, G.W. 1909 (rev. 1972). Indian basketry. Dover Publications, Inc., New York, New York. 271 pp. Kindscher, K. 1992. Medicinal wild plants of the prairie. An ethnobotanical guide. University Press of Kansas. 340 pp. Kindscher, K. 1987. Edible wild plants of the prairie. An ethnobotanical guide. University Press of Kansas. 276 pp. Knopf, F.I. & F.B. Samson 1994. Scale perspectives on avian diversity in western riparian ecosystems. Conservation Biology 8(3):669-676. Marlow, C.B. & T.M. Pogacnik 1985. Time of grazing and cattle-induced damage to streambanks. Pages 279-284. IN Johnson, R.R., C.D. Ziebell, D.R. Patton, P.F. Folliott, and R.H. Hamre. (Tech. Coords.) Riparian ecosystems and their management: Reconciling conflicting uses. Proc. First North Am. Riparian Conf., USDA, FS, Gen. Tech. Rep. RM-120. 523 pp. Martin, A.C., H.S. Zim, & A.L. Nelson 1951. American wildlife and plants. A guide to wildlife food habits. Dover Publications, Inc., New York, New York. 500 pp. Mason, O.T. 1988. American Indian basketry. Dover Publications, Inc., New York, New York. McGregor, R.L., T.M. Barkley, R.E. Brooks, &E.K. Schofield (eds.) 1976. Flora of the Great Plains. Great Plains Flora Association. University Press of Kansas. 1402 pp. Moore, M. 1979. Medicinal plants of the mountain west. Museum of New Mexico Press. 200 pp. Smith, H.H. 1928. Ethnobotany of the Meskwaki Indians. Bulletin of the Public Museum of the City of Milwaukee 4(2):175-326. Stephens, H.A. 1973. Woody plants of the north central plains. The University Press of Kansas. 530 pp. Stevens, L.E., B.T. Brown, J.M. Simpson, & R.R. Johnson 1977. The importance of riparian habitat to migrating birds, pp. 156-164. IN Johnson, R.R. & D.A. Jones (Tech. Coords.). Importance, preservation and management of riparian habitat: a symposium. USDA, Forest Service, Gen. Tech. Report RM-43, Rocky Mountain Forest and
Range
Experiment Station, Fort Collins, Colorado. 217 pp. Tilford, G.L. 1997. Edible and medicinal plants of the west. Mountain Press Publishing Company, Missoula, Montana. USDA, NRCS 1995. Midwestern wetland flora. Wetland Science Institute, Laurel, Maryland. USDA, NRCS 2000. The PLANTS database. Version: 000321. <http://plants.usda.gov>. National Plant Data Center, Baton Rouge, Louisiana. Vestal, P.A. & R.E. Schultes 1939. The economic botany of the Kiowa Indians. Botanical Museum, Harvard University, Cambridge, Massachusetts.
Plant Traits
Growth Requirements
| Temperature, Minimum (°F) | -33 |
|---|---|
| Adapted to Coarse Textured Soils | Yes |
| Adapted to Fine Textured Soils | No |
| Adapted to Medium Textured Soils | Yes |
| Anaerobic Tolerance | Medium |
| CaCO3 Tolerance | Medium |
| Cold Stratification Required | Yes |
| Drought Tolerance | Low |
| Fertility Requirement | Medium |
| Fire Tolerance | High |
| Frost Free Days, Minimum | 130 |
| Hedge Tolerance | Low |
| Moisture Use | High |
| pH, Maximum | 8.0 |
| pH, Minimum | 6.0 |
| Planting Density per Acre, Maxim | 700 |
| Planting Density per Acre, Minim | 170 |
| Precipitation, Maximum | 60 |
| Precipitation, Minimum | 24 |
| Root Depth, Minimum (inches) | 30 |
| Salinity Tolerance | None |
| Shade Tolerance | Intolerant |
Morphology/Physiology
| Bloat | None |
|---|---|
| Toxicity | None |
| Resprout Ability | Yes |
| Shape and Orientation | Irregular |
| Active Growth Period | Spring and Summer |
| C:N Ratio | Medium |
| Coppice Potential | No |
| Fall Conspicuous | No |
| Fire Resistant | No |
| Flower Color | White |
| Flower Conspicuous | No |
| Foliage Color | Green |
| Foliage Porosity Summer | Dense |
| Foliage Porosity Winter | Porous |
| Fruit/Seed Conspicuous | No |
| Nitrogen Fixation | None |
| Low Growing Grass | No |
| Lifespan | Short |
| Leaf Retention | No |
| Known Allelopath | No |
| Height, Mature (feet) | 60.0 |
| Height at 20 Years, Maximum (fee | 45 |
| Growth Rate | Rapid |
| Growth Form | Multiple Stem |
| Foliage Texture | Medium |
Reproduction
| Vegetative Spread Rate | None |
|---|---|
| Small Grain | No |
| Seedling Vigor | Low |
| Seed Spread Rate | Moderate |
| Seed per Pound | 2600000 |
| Fruit/Seed Persistence | No |
| Propagated by Tubers | No |
| Propagated by Sprigs | No |
| Propagated by Sod | No |
| Propagated by Seed | No |
| Propagated by Corm | No |
| Propagated by Cuttings | Yes |
| Bloom Period | Late Spring |
| Commercial Availability | Routinely Available |
| Fruit/Seed Abundance | High |
| Fruit/Seed Period Begin | Spring |
| Fruit/Seed Period End | Spring |
| Propagated by Bare Root | Yes |
| Propagated by Bulb | No |
| Propagated by Container | Yes |
Suitability/Use
| Veneer Product | No |
|---|---|
| Pulpwood Product | No |
| Protein Potential | Low |
| Post Product | No |
| Palatable Human | No |
| Palatable Graze Animal | Medium |
| Palatable Browse Animal | Medium |
| Nursery Stock Product | Yes |
| Naval Store Product | No |
| Lumber Product | No |
| Fuelwood Product | Low |
| Fodder Product | No |
| Christmas Tree Product | No |
| Berry/Nut/Seed Product | No |