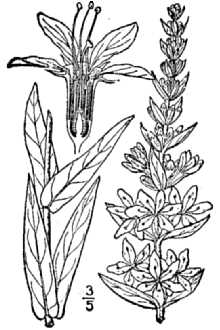
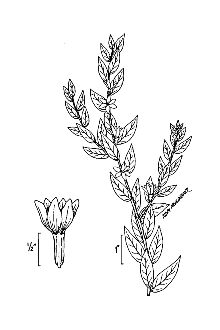
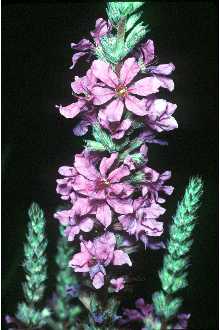
Lythrum salicaria L. var. gracilior Turcz.
Scientific Name: Lythrum salicaria L. var. gracilior Turcz.
| General Information | |
|---|---|
| Usda Symbol | LYSAG |
| Group | Dicot |
| Life Cycle | Perennial |
| Growth Habits | Forb/herbSubshrub, |
| Native Locations | LYSAG |
Plant Guide
Alternate Names
purple loosestrife, spiked lythrum, salicaire, bouquet violet , Use soil moisture sensors to measure the soil moisture of Lythrum salicaria L. var. gracilior Turcz..
Uses
Noxious and highly invasive. Ethnobotanic: Immigrants might have deliberately introduced L. salicaria for its value as a medicinal herb in treating diarrhea, dysentery, bleeding wounds, ulcers, and sores, for ornamental purposes, or as a source of nectar and pollen for beekeepers (Hayes 1979; Jones 1976; Malecki et al. 1993; Stuckey 1980). In states where it is permitted, purple loosestrife continues to be promoted by horticulturists for its beauty as a landscape plant and for bee-forage. Purple loosestrife has been of interest to beekeepers because of its nectar and pollen production. However, honey produced from it is apparently of marginal quality (Feller-Demalsy & Parent 1989). Horticultural: Horticultural cultivars of purple loosestrife (Lythrum spp.) were developed in the mid-1900s for use as ornamentals. Initially, these were thought to be sterile, and therefore safe for horticultural use. Recently, under greenhouse conditions, experimental crosses between several cultivars and wild purple loosestrife and the native L. alatum produced hybrids that were highly fertile (Ottenbreit 1991; Ottenbreit & Staniforth 1994). Comparable, subsequent experiments performed under field conditions produced similar results, suggesting that cultivars of purple loosestrife can contribute viable seeds and pollen that can contribute to the spread of purple loosestrife (Lindgren & Clay 1993). Ottenbreit & Staniforth (1994) indicate that such results suggest the need to prohibit cultivars of this species. Robert Mohlenbrock USDA, NRCS, Wetland Science Institute @ PLANTS Noxiousness: Purple loosestrife grows most abundantly in parts of Canada, the northeastern United States, the Midwest, and in scattered locations in the West. Although this species tolerates a wide variety of soil conditions, its typical habitat includes cattail marshes, sedge meadows, and bogs. It also occurs along ditch, stream, and riverbanks, lake shores, and other wet areas. In such habitats, purple loosestrife forms dense, monospecific stands that can grow to thousands of acres in size, displacing native, sometimes rare, plant species and eliminating open water habitat. The loss of native species and habitat diversity is a significant threat to wildlife, including birds, amphibians, and butterflies, that depend on wetlands for food and shelter. Purple loosestrife monocultures also cause agricultural loss of wetland pastures and hay meadows by replacing more palatable native grasses and sedges (Mal et al. 1992; Thompson et al. 1987). Having a noxious weed designation in some states prohibit its importation and distribution, but it is readily available commercially in many parts of the country. Lythrum salicaria has been labeled the “purple plague." because of its epidemic devastation to natural communities. The species is included on the Nature Conservancy’s list of “America’s Least Wanted -The Dirty Dozen” (Flack & Furlow 1996). Impact/Vectors: Naturalized purple loosestrife was relatively obscure from the time of its introduction into North America in the early 1800s (Pursh 1814) until 1930, when a significant increase in populations invading wetlands and pastures was documented (Strefeler et al. 1996b). Reasons for the apparent sudden colonization and spread of this species include the disturbance of natural systems by human activities including agricultural settlement, construction of transport routes such as canals, highways, and perhaps, nutrient increases to inland waters (Mal et al. 1992; Malecki et al. 1993). Absence of natural enemies and ornamental use are other possible causes for purple loosestrife’s rapid expansion in North America (Thompson et al, 1987). Recently created irrigation systems in many western states have supported further establishment and spread of L. salicaria (Malecki et al. 1993). The acquisition of adaptive characteristics from native species of Lythrum may have enhanced purple loosestrife’s invasive success. It will hybridize with Lythrum alatum, a widespread, native North American species, in natural settings. Under certain circumstances fertile hybrids are produced that can cross with weedy purple loosestrife. Such interspecific hybrids could serve as a “hybrid bridge” for the transfer of adaptive traits from native L. alatum into weedy populations of purple loosestrife (Anderson & Ascher 1993; Strefeler et al. 1996b). North American naturalized populations of purple loosestrife often form monospecific stands, whereas, in its native Eurasian habitat the species comprises 1-4% of the vegetative cover (Batra et al. 1986; Strefeler et al. 1996b). Purple loosestrife causes annual wetland losses of about 190,000 hectares in the United States (Thompson et al. 1987; Mal et al. 1997). The species is most abundant in the Midwest and Northeast where it infests about 8,100 hectares in Minnesota, 12,000 ha in Wisconsin, over 12,000 ha in Ohio, and a larger area in New York State. Recent distributional surveys document the occurrence of monocultures in every county in Connecticut, where it has been found in 163 wetland locations (Ellis and Weaver 1996; Ellis 1996). At the Effigy Mounds National Monument (EFMO), combined populations of purple loosestrife cover an area of 5 to 10 hectares growing in regularly disturbed sites. This species has a major visual impact on the vegetation of EFMO, and it has the potential to invade and replace native communities endangering the areas' primary resources. (Butterfield et al. 1996). In response to the alarming spread of this exotic species, at least 13 states (e.g., Minnesota, Illinois, Indiana, Ohio, Washington, and Wisconsin) have passed legislation restricting or prohibiting its importation and distribution (Malecki et al. 1993; Strefeler et al. 1996b). Numerous studies demonstrate the aggressive and competitive nature of purple loosestrife. Fernald (1940) reported a loss of native plant diversity in the St. Lawrence River floodplain following the invasion of purple loosestrife and another exotic, Butomus umbellatus L. Gaudet and Keddy (1988) report declining growth for 44 native wetland species after the establishment of Lythrum. Among the species tested, Keddy (1990) found that purple loosestrife was the most competitive. His hierarchical rank, arranged from most to least competitive, illustrates the dominance of this invasive weed over many common natives: Lythrum>Cyperus>Juncus> Eleocharis> Mimulus>Verbena. In the Hamilton Marshes adjacent to the Delaware River, annual above-ground production of L. salicaria far exceeded all other plant species’ production combined. Purple loosestrife provides little food, poor cover, and few nesting materials for wildlife (Mann 1991). Waterfowl nesting becomes more difficult as clumps of L. salicaria restrict access to open water and offer concealing passageways for predators such as foxes and raccoons (Mal et al. 1992). Non-game species, including black terns and marsh wrens, also lose nesting sites when purple loosestrife infests their normal habitats. Balogh and Bookhout (1989a) report that dense stands of purple loosestrife provide poor waterfowl and muskrat habitat. Red-wing blackbirds appear to be the only species to cope with changes in wetlands caused by purple loosestrife (Balogh and Bookhout 1989a). In many areas where L. salicaria populations have increased, wildlife species have declined. While some studies may fail to demonstrate cause and affect relationship, they firmly establish circumstantial evidence implicating that Lythrum’s invasion is responsible for major changes in wetland communities (Mal et al. 1992). Purple loosestrife prefers moist, highly organic soils but can tolerate a wide range of conditions. It grows on calcareous to acidic soils, can withstand shallow flooding, and tolerates up to 50% shade. Purple loosestrife has low nutrient requirements and can withstand nutrient poor sites. Under experimental, nutrient-deficient conditions, the root/shoot ratio increased and provided purple loosestrife with a competitive advantage over the native species Epilobium hirsutum. Survival and growth of L. salicaria was greatly improved by fertilizer treatment and greater spacing between plants. Such results suggest that excessive use of fertilizers and the release of phosphates, nitrates, and ammonia into the environment has enhanced the success of Lythrum (Mal et al., 1992; Shamsi and Whitehead, 1977a and b). Purple loosestrife flowers from July until September or October. Flowering occurs 8-10 weeks after initial spring growth. The lowermost flowers of the inflorescence open first and flowering progresses upward. The capsules mature in the same sequence and the lowermost will ripen and disperse its seeds while flowering is still occurring further up the inflorescence (Butterfield et al. 1996). Thompson et al. (1987) estimated that on average, a mature plant produces about 2,700,000 seeds annually. Purple loosestrife seeds are mostly dispersed by water, but wind and mud adhering to wildlife, livestock, vehicle tires, boats, and people serve also as agent. Seeds are relatively long-lived, retaining 80% viability after 2-3 years of submergence (Malecki 1990). Welling & Becker (1990) investigated seed bank dynamics in three wetland sites in Minnesota and noted a mean density of 410,000 seeds per square meter in the top 5 cm of soil, which was more than all other species combined. Spring-germinated seedlings have a higher survival rate than summer-germinated seedlings. Seedlings that germinate in the spring will flower the first year, whereas, summer-germinated seedlings develop only five or six pairs of leaves before the end of the growing season. Since its seeds are small, weighing about 0.06 mg each and carry little food reserves, germination must occur under conditions where photosynthesis can occur immediately. A strong taproot develops quickly in seedlings and persists throughout the life of the plant. The aerial shoots die in the fall and new shoots arise the following spring from buds on the rootstocks. Shoots destroyed by fire, herbicides, or mechanical removal can also regenerate from the rootstock. As plants mature, they produce more and more aerial shoots forming very dense clumps of growth. Purple loosestrife can spread vegetatively by resprouting from stem cuttings and from regeneration of pieces of root stock (Mal et al. 1992). Rhizomatous growth is insignificant in purple loosestrife (Shamsi & Whitehead 1974a; Thompson et al. 1987).
Status
Please consult the PLANTS Web site and your State Department of Natural Resources for this plant’s current status, such as, state noxious status, and wetland indicator values.
Description
General: Loosestrife Family (Lythraceae). Purple loosestrife is an erect perennial herb that grows up to 2.5 m tall, develops a strong taproot, and may have up to 50 stems arising from its base. Its 50 stems are four-angled and glabrous to pubescent. Its leaves are sessile, opposite or whorled, lanceolate (2-10 cm long and 5-15 mm wide), with rounded to cordate bases. Leaf margins are entire. Leaf surfaces are pubescent. Each inflorescence is spike-like (1-4 dm long), and each plant may have numerous inflorescences. The calyx and corolla are fused to form a floral tube (also called a hypanthium) that is cylindrical (4-6 mm long), greenish, and 8-12 nerved. Typically the calyx lobes are narrow and thread-like, six in number, and less than half the length of the petals. The showy corolla (up to 2 cm across) is rose-purple and consists of five to seven petals. Twelve stamens are typical for each flower. Individual plants may have flowers of three different types classified according to stylar length as short, medium, and long. The short-styled type has long and medium length stamens, the medium type has long and short stamens, and the long-styled has medium to short stamens. The fruit is a capsule about 2 mm in diameter and 3-4 mm long with many small, ovoid dust-like seeds (< 1 mm long). Mal et al., 1992, provide a detailed morphological description for L. salicaria. The authors also give details of the tristylous features of this species, as well as an account of its pollen structure and chromosome numbers. The plant’s habit, vegetative, and reproductive structures are illustrated with line drawings. Other species of Lythrum that grow in the United States have 1-2 flowers in each leaf-like inflorescence bract and eight or fewer stamens compared to L. salicaria, which has more than two flowers per bract and typically twelve stamens per flower. Lythrum virgatum, another species introduced from Europe closely resembles L. salicaria, but differs in being glabrous (lacking plant hairs), and having narrow leaf bases. The latter two species interbreed freely producing fertile offspring, and some taxonomists (Rendall 1989) consider them to be a single species. Distribution: Purple loosestrife is a hardy perennial herb with stunning spikes of purple flowers. A native of Eurasia, it was introduced to North America in the early 1800's where it first appeared in ballast heaps of eastern harbors (Stuckey 1980). Most likely seeds were transported as contaminants in the ballast or possibly attached to raw wool or sheep imported from Europe (Cole, 1926; Thompson et al., 1987). The native range of L. salicaria is thought to extend from Great Britain to central Russia from near the 65th parallel to North Africa. It also occurs in Japan, Korea, and the northern Himalayan region. The species has been introduced to Australia, Tasmania, and New Zealand. Since its introduction to North America, this alien plant has spread rapidly into Canada, and throughout most of the United States where it has been reported from all states except Alaska, Florida, Louisiana, and South Carolina. Several factors have contributed to the spread of purple loosestrife such as its potential for rapid growth, its enormous reproductive capacity, lack of natural diseases or predators, its use as an ornamental, and for bee forage (Mal et al. 1992). For current U.S. distribution, please consult the Plant Profile page for this species on the PLANTS Web site.
Control
Please contact your local agricultural extension specialist or county weed specialist to learn what works best in your area and how to use it safely. Always read label and safety instructions for each control method. Trade names and control measures appear in this document only to provide specific information. USDA, NRCS does not guarantee or warranty the products and control methods named, and other products may be equally effective. An important consideration in controlling purple loosestrife is its prolific seed production, the ease with which seeds are dispersed, and their ability to remain viable for several years. Also, this plant can spread vegetatively by resprouting from stem and rootstock cuttings. Other considerations in selecting control methods are their detrimental effects on native species and the possibility for reinvasion by purple loosestrife or other exotic species. In addition, native plants of similar appearance should not be subjected to control. Purple loosestrife may superficially resemble plants of the mint family or species of the genera Epilobium and Liatris. Proper identification is an important consideration in controlling exotic loosestrife. In natural areas, it may be more feasible to contain populations of purple loosestrife than control them. Large populations extending over one hectare or more will be difficult to eradicate. Containing them may be more feasible. Removing plants or applying herbicides to ones extending beyond the main population can accomplish this. If loosestrife cannot be eradicated, efforts should then concentrate on keeping it from invading the highest quality areas (Butterfield et al., 1996. Manual, Mechanical, and Replacement: Mowing, burning, and flooding are largely ineffective. Cutting followed by flooding so that cut plant stalks are completely immersed has shown some success. However, flooding may encourage the spread of purple loosestrife seed present in the soil and may result in the regeneration of new plants from stem fragments. Mature plants can withstand short-term immersion. Burning is largely ineffective and it may also stress native plants and subsequently enhance loosestrifes’ competitive advantage (Butterfield et al., 1996). Hand removal is effective for small populations and isolated plants. Younger plants (one to two years old) can be pulled by hand. Plants should be removed, prior to seed set, with minimal disturbance to the soil. Removal after seed-set will scatter the seeds. The entire rootstock must be pulled out because of the potential for regeneration from root fragments. A hand cultivator or similar implement will be helpful for older plants, especially those in deep organic soils. Uprooted plants and broken stems need to be removed from the site since such fragments can re-sprout. Bagging plants for removal will prevent their spread along the exit route. Follow-up treatments are recommended for three years after plants are removed. Clothing and equipment used during plant removal should be cleaned to remove contaminating seeds. Replacement control has been attempted in several wildlife refuges. Research has shown that Japanese millet (Echinochloa frumentacea Link) seedlings outcompete purple loosestrife seedlings. The millet must be planted immediately after marsh drawdown and replanted each year because it does not regenerate well. Replacement seeding trials using native pale smartweed (Polygonum lapathifolium L.) showed that it also out-competed purple loosestrife. Replacement methods have obvious limited application in natural areas, but they may provide control of loosestrife populations on bordering property (Butterfield et al. 1996). Herbicide Control: Various chemical treatments have been used on purple loosestrife with varying success. Many herbicides are not specific to purple loosestrife and may not be specifically licensed for such use. Label directions for application and use according to local, state, and federal regulations must always be observed. In areas with populations exceeding 100 plants (up to 1.6 ha in size) where hand-pulling is not feasible, application of a glyphosate herbicide to individual purple loosestrife plants provides effective control Glyphosate is available under the trade names Roundup® and Rodeo®. Rodeo is registered for use over open water and is the most commonly used herbicide to control purple loosestrife. Glyphosate is nonselective and can kill desirable plants associated with loosestrife if applied carelessly. Application to the tops of plants alone can be effective and limits exposure of non-target species (Butterfield et al. 1996). Herbicide treatment should be conducted as early as possible during the manufacturer's recommended time of application in order to kill the plants and prevent seed production. Application is most effective when plants have just begun flowering. Timing is important because seed set can occur if plants are in mid- to late flower. Where possible, the flower heads should be cut, bagged, and removed from the site prior to application to prevent seed set. Rodeo applied as a 1.5% solution (2 oz. Rodeo/gallon clean water) with the addition of a wetting agent, as specified on the label has been shown to provide control. Another option, which may be more effective, is to apply glyphosate twice during the growing season. The plants should be sprayed as described above when flowering has just started and a second time two to three weeks later (Butterfield et al. 1996). Application of ghyphosate from a vehicle-mounted sprayer is generally necessary in areas with extensive stands of purple loosestrife. The most effective control can be achieved by beginning treatment at the periphery of large patches and working toward the center in successive years. This technique allows native vegetation to re-invade the treated area as the loosestrife in eliminated (Butterfield et al. 1996). A combination of 2,4-D and Banvel® (dicamba) has been used on a limited basis. This formulation is broadleaf specific and apparently would not hurt the dominants if sprayed in a cattail marsh or communities dominated by rushes, sedges, and grasses. Spraying produces good control once loosestrife has reached 10-15% of its mature growth. Treatment is more effective if repeated once during the growing season (Butterfield et al. 1996). Biological Control: Several biological control agents have the potential to aid in the control of purple loosestrife. Of 120 species of phytophagous insects associated with purple loosestrife in its natural range in Europe, 14 species were considered host-specific to the target plant. From this group, six species have been selected as the most promising for biological control. These species were a root-mining weevil, Hylobius transversovittatus Goeze, which attacks the main storage tissue of purple loosestrife; two leaf-eating beetles, Galerucella calmariensis L., and G. pusilla Duftschmid, which are capable of completely defoliating the plant; two flower-feeding beetles, Nanophyes marmoratus Goeze and N. brevis Boheman, which severely reduce seed production; and a gall midge, Bayeriola salicariae Kieffer, which similarly reduces seed production by attacking the flower buds. Five of the six species are found throughout its range in Europe and the sixth, N. brevis, is restricted to southern Europe (Malecki et al. 1993; Weedin et al. 1996). The most promising insects appear to be the root-mining weevil, H. transversovittatus, and the two leaf-eating beetles, G. calmariensis and G. pusilla, because of their broad geographic ranges and the amount of damage done to the host plant. In June of 1992, all three species were approved by USDA, APHIS for introduction into the United States. The insects were released in New York, Pennsylvania, Maryland, Virginia, Minnesota, Oregon, and Washington. Releases were also approved in Canada (Malecki et al. 1993). The two Galerucella species successfully over-wintered and began oviposition at all release sites. The other species, H. transversovittatus, was proving more difficult to establish, because of its long life cycle and low fecundity. The investigators predict that all three species will become established throughout the North American range of purple loosestrife. Furthermore, H. transversovittatus is expected to have the greatest negative impact to L. salicaria. However, a combination of various phytophagous insects will provide greater control than any one species. Control of purple loosestrife will be achieved more rapidly in mixed plant communities where competition for space and nutrients is greater. A reduction in the abundance of purple loosestrife to approximately 10% of its current level over about 90% of its range is expected (Malecki et al. 1993). In order to evaluate the potential of fungus pathogens to control purple loosestrife, a survey was conducted on fungi associated with that plant. During the three year study, 5265 fungal isolates were obtained. Thirty-one taxa were found that had not previously been reported from purple loosestrife. Tests for the pathogenicity to purple loosestrife are being tested (Nyvall 1995). Illustrations and Photographs: Gleason, H. A. 1952. Illustrated flora of the northeastern United States and adjacent Canada. Lancaster Press, Inc., Lancaster, Pennsylvania. (line drawing, Vol. 3, p. 53). Mal, T.K., J. Lovett-Doust, L. Lovett-Doust, & G.A. Mulligan 1992. The biology of Canadian weeds. 100. Lythrum salicaria. Can. J. Plant Sci. Rev. Can. Phytotech. 72(4):1305-1330. Agricultural Institute of Canada, Ottawa, Ontario, Canada. (detailed line illustrations of the whole plant and flower morphs). O'Neil, P. 1992. Variation in male and female reproductive success among floral morphs in the tristylous plant Lythrum salicaria (Lythraceae). American J. of Botany 79(9):1024-1030. Botanical Society of America, Columbus, Ohio (detailed illustration of the three (tristyly) flower morphs). Radford, A. E., H. E. Ahles, & C. R. Bell 1968. Manual of the vascular flora of the Carolinas. University of North Carolina Press, Chapel Hill, North Carolina. (small line drawing, p. 831). Randall, J. M. & J. Marinelli 1996. Invasive plants, weeds of the global garden. Brooklyn Botanic Garden, Handbook #149, Brooklyn, New York. 99 p. (photographs of plants in flower, front cover, p. 5 and p. 81; infested habitat, p. 5). USDA, NRCS 2000. The PLANTS database. Version: 000412. <http://plants.usda.gov>. National Plant Data Center, Baton Rouge, Louisiana. USDA, NRCS 2000. Wetland flora CD-ROM. <http://www.pwrc.usgs.gov/wli/wetprods.htm>. Wetland Science Institute, Laurel, Maryland.
References
Anderson, N.O. & P.D. Ascher 1993. Male and female fertility of loosestrife (Lythrum) cultivars. Journal of the American Horticulture Society 118(6):851-858. Balogh, G.R. & T.A. Bookhout 1989a. Purple loosestrife (Lythrum salicaria) in Ohio's Lake Erie marshes. Ohio Journal of Science 89(3):62-64. maps. Batra, S.W.T., D. Schroeder, P.E. Boldt, & W. Mendel 1986. Insects associated with purple loosestrife (Lythrum salicaria L.) in Europe. Proc. Entomol. Soc. Wash., 88(4):748-759, Washington, D.C. Blossey, B. 1993. Herbivory below ground and biological weed control: life history of a root-boring weevil on purple loosestrife. Oecologia 94(3):380-387. Blossey, B. 1995. A comparison of various approaches for evaluating potential biological control agents using insects on Lythrum salicaria. Biological Control 5(2):113-122. Blossey, B. & R.U. Ehlers 1991. Entomopathogenic nematodes (Heterorhabditis spp. and Steinernema anomali) as potential antagonists of the biological weed control agent Hylobius transversovittatus (Coleoptera: Curculionidae). J. Invertebrate Pathology 58(3):453-454. Blossey, B. & R. Notzold 1995. Evolution of increased competitive ability in invasive nonindigenous plants: a hypothesis. J. Ecology 83(5):887-889. Blossey, B. & D. Schroeder 1995. Host specificity of three potential biological weed control agents attacking flowers and seeds of Lythrum salicaria (purple loosestrife). Biological Control 5(1):47-53. Blossey, B., D. Schroeder, S.D. Hight, & R.A. Malecki 1994a. Host specificity and environmental impact of two leaf beetles (Galerucella calmariensis and G. pusilla) for biological control of purple loosestrife (Lythrum salicaria). Weed Science 42(1):134-140. Blossey, B., D. Schroeder, S.D. Hight, & R.A. Malecki 1994b. Host specificity and environmental impact of the weevil Hylobius transversovittatus, a biological control agent of purple loosestrife (Lythrum salicaria). Weed Science 42(1):128-133. Brungardt, S. [1992.] Wisdom of a flower ban supported by research. Minn. Sci. Agric. Exp. Stn. Univ. Minn. 47(1):4-5. Butterfield, C., J. Stubbendieck, & J. Stumpf 1996. Species abstracts of highly disruptive exotic plants. Version: 16JUL97. Northern Prairie
Wildlife
Research Center Home Page, Jamestown, North Dakota. http://www.npsc.nbs.gov/resource/othrdata/plntguid/species/lythsali.htm. Cole, A.H. 1926. The American manufacture. Vol. 1. Harvard University Press, Cambridge, Massachusetts. Cornell University, Educational Television Center and Media Services 1996. Restoring the balance: biological control of purple loosestrife. Ithaca, New York. 1 videocassette (28 min.). Ellis, D. R. 1996. Biological control of purple loosestrife in Connecticut. 1996 Summary Report. <http://www.ceris.purdue.edu/napis/pests/pls/index.html>. (15 May 1997). Ellis, D.R. & J.S. Weaver 1996. Purple loosestrife: Survey and biological control. 1995 Summary Report, USDA, APHIS, PPQ, Cooperative Agricultural Pest Survey (CAPS). 9 pp Feller Demalsy, M.J. & J. Parent 1989. Analyse pollinique des miels de l’Ontario, Canada. Apidologie 20:127-138. Fernald, M.L. 1940. The problem of conserving rare native plants. Annual Report, Smithsonian Institution (1939):375-391. Flack, S. & E. Furlow 1996. America's least wanted "purple plague," "green cancer" and 10 other ruthless environmental thugs. Nature Conservancy Magazine 46(6) November/December. <http://www.tnc.org/news/magazine/nov_dec/index.html>. Gabor, T.S., & H.R. Murkin 1990. Effects of clipping purple loosestrife seedlings during a simulated wetland drawdown. Journal of Aquatic Plant Management 28:98-100. Gardner, S.C. & C.E. Grue 1996. Effects of Rodeo and Garlon 3A on nontarget wetland species in central Washington. Environmental Toxicolog. Chem. 15(4): 441-451. Gaudet, C.L. & P.A. Keddy 1988. A comparative approach to predicting competitive ability from plant traits. Nature 334(6179):242-243. Gaudet, C.L. & P.A. Keddy 1995. Competitive performance and species distribution in shoreline plant communities: a comparative approach. Ecology 76(1):280-291. Hayes, B. 1979. Purple loosestrife - The wetlands honey plant. American Bee Journal 119:382-383. Heidorn, R., & B. Anderson 1991. Vegetation management guideline: purple loosestrife (Lythrum salicaria L.). Natural Areas Journal 11:172-173. Heuch, I. 1979. The effect of partial self-fertilization on type frequencies in heterostylous plants Lythrum salicaria. Annals of Botany 44(5):611-616. ill. Hight, S.D., B. Blossey, J. Laing & R. DeClerck-Floate 1995. Establishment of insect biological control agents from Europe against Lythrum salicaria in North America. Environmental Entomology 24(4):967-977. Jones, M.A. 1976. Destination America. Holt, Rinehart & Winston, New York, New York. Katovich, E.J.S., R.L. Becker, & B.D. Kinkaid 1996. Influence of nontarget neighbors and spray volume on retention and efficacy of triclopyr in purple loosestrife (Lythrum salicaria). Weed Science 44(1):143-147. Keddy, C. 1988. A review of Lythrum salicaria (purple loosestrife) ecology and management: The urgency for management in Ontario. Natural Heritage League, Ottawa, Ontario, Canada. 34 pp. Keddy, C. 1990. The use of functional as opposed to phylogenetic systematics: a first step in predictive community ecology. IN: S. Kawano, ed. Biological approaches and evolutionary trends in plants. Academic Press, London, U.K. Kok, L.T., T.J. McAvoy, R.A. Malecki, S.D. Hight, J.J. Drea, & J.R. Coulson 1992. Host specificity tests of Hylobius transversovittatus Goeze (Coleoptera: Curculionidae), a potential biological control agent of purple loosestrife, Lythrum salicaria L. (Lythraceae). Biological Control 2:1-8. Kok, L.T., T.J. McAvoy, R.A. Malecki, S.D. Hight, J.J. Drea, & J.R. Coulson 1992. Host specificity tests of Galerucella calmariensis (L.) and G. pusilla (Duft.) (Coleoptera Chrysomelidae), potential biological control agents of purple loosestrife, Lythrum salicaria L. (Lythraceae). Biological Control 2:282-290. Lindgren, C.J. & R.T. Clay 1993. Fertility of 'Morden Pink' Lythrum virgatum L. transplanted into wild stands of L. salicaria L. in Manitoba. HortScience 28(9):954. Mal, T.K., J. Lovett-Doust, L. Lovett-Doust, & G.A. Mulligan 1992. The biology of Canadian weeds. 100. Lythrum salicaria. Can. J. Plant Sci. Rev. Can. Phytotech. 72(4):1305-1330. Mal, T.K., J. Lovett-Doust, & L. Lovett-Doust 1997. Time-dependent competitive displacement of Typha angustifolia by Lythrum salicaria. Oikos 79:26-33. Malecki, R.A., B. Blossey, S.D. Hight, D. Schroeder, L.T. Kok, & J.R. Coulson 1993. Biological control of purple loosestrife. BioScience 43:680-686. Malecki, R. 1990. Research update-Biological control of purple loosestrife. Report of New York Cooperative Fish and Wildlife Research Unit, Cornell University, Ithaca, New York. 4pp + 2 figs. Malecki, R. 1994. Insect biological weed control: an important and underutilized management tool for maintaining native plant communities threatened by exotic plant introductions. Trans. North. Am. Wildl. Nat. Resoures Conf. (59th) p. 400-404. Wildlife Management Institute, Washington, DC. Mann, H. 1991. Purple loosestrife: A botanical dilemma. The Osprey 22:67-77. Notestein, A. 1987. Purple loosestrife managed with herbicides at Horicon National Wildlife Refuge (Wisconsin). Restoration and Management Notes 5:91. Nyvall, R.F. 1995. Fungi associated with purple loosestrife (Lythrum salicaria) in Minnesota. Mycologia 87(4):501-506. Nyvall, R.F. & A. Hu 1997. Laboratory evaluation of indigenous North American fungi for biological control of purple loosestrife.
Biological Control
8(1):37-42. O'Neil, P. 1997. Natural selection on genetically correlated phenological characters in Lythrum salicaria L. (Lythraceae). Evolution 51(1):267-274. Ottenbreit, K.A. 1991. The distribution, reproductive biology, and morphology of Lythrum species, hybrids and cultivars in Manitoba. M.Sc. thesis, Department of Botany, University of Manitoba, Winnipeg, Manitoba, Canada. Ottenbreit, K.A. & R.J. Staniforth 1994. Crossability of naturalized and cultivated Lythrum taxa. Canadian Journal of Botany 72(3):337-341. Pursh, F. 1814. Flora Americae Septentrionalis (or systematic arrangement and description of the plants of North America). White, Cochrane, and Co., London, England. Rendall, J. 1989. The Lythrum story: A new chapter. Minnesota Horticulturist 117:22-24. Shamsi, S.R.A., & F.H. Whitehead 1974a. Comparative eco-physiology of Epilobium hirsutum L. and Lythrum salicaria L. I. IN General biology, distribution, and germination. Journal of Ecology 62:279-290. Shamsi, S.R.A., & F.H. Whitehead 1974b. Comparative eco-physiology of Epilobium hirsutum L. and Lythrum salicaria L. II. IN Growth and development in relation to light. Journal of Ecology 62:631-645. Shamsi, S.R.A., & F.H. Whitehead 1977a. Comparative eco-physiology of Epilobium hirsutum L. and Lythrum salicaria L. III. IN Mineral nutrition. Journal of Ecology 65:55-70. Shamsi, S.R.A., & F.H. Whitehead 1977b. Comparative eco-physiology of Epilobium hirsutum L. and Lythrum salicaria L. IV. IN Effects of temperature and inter-specific competition and concluding discussion. Journal of Ecology 65:71-84. Shamsi, S.R.A. 1976. Effect of a light-break on the growth and development of Epilobium hirsutum and Lythrum salicaria in short photoperiods. Annals of Botany 40(165):153-162. Strefeler, M.S., E. Darmo, R.L. Becker, & E.J. Katovich 1996a. Isozyme variation in cultivars of purple loosestrife (Lythrum sp.). HortScience 31(2):279-282. Strefeler, M.S., E. Darmo, R.L. Becker, & E.J. Katovich 1996b. Isozyme characterization of genetic diversity in Minnesota populations of purple loosestrife, Lythrum salicaria (Lythraceaae). American Journal of Botany 83(3):265-273. Stuckey, R.L. 1980. Distributional history of Lythrum salicaria (purple loosestrife) in North America. Bartonia 17(47):3-20. Thompson, D.Q. 1991. History of purple loosestrife (Lythrum salicaria L.) biological control effects. Natural Areas Journal 11:148-150. Thompson, D.Q., R.L. Stuckey, & E.B. Thompson 1987. Spread, impact, and control of purple loosestrife (Lythrum salicaria) in North American wetlands. Research Report No. 2. USDI, Fish and Wildlife Service, Washington, DC. Twolan-Strutt, L. & P.A. Keddy 1996. Above- and below-ground competition intensity in two contrasting wetland plant communities. Ecology 77(1):259-270. Voegtlin, D.J. 1995. Potential of Myzus lythri (Homoptera: Aphididae) to influence growth and development of Lythrum salicaria (Myrtiflorae: Lythraceae). Environmental Entomology 24(3):724-729. Weeden, C. R, A. M. Shelton, & M. P. Hoffmann, Eds. 1996. Biological control: A guide to natural enemies in North America. Cornell University. (http://www.nysaes.cornell.edu/ent/biocontrol/weedfeeders/galerucella.html; http://www.nysaes.cornell.edu/ent/biocontrol/ weedfeeders/hylobius.html). Welling, C.H. & R.L. Becker 1990. Seed bank dynamics of Lythrum salicaria L.: implications for control of this species in North America. Aquatic Botany 38:303-309. Wilcox, D.A., M.K. Seeling, & K.R. Edwards 1986. Ecology and management potential for purple loosestrife (Lythrum salicaria). IN The George Wright Society conference on science in the National Parks. USDI, National Park Service, Washington, DC. Wilcox, D.A. 1989. Migration and control of purple loosestrife (Lythrum salicaria L.) along highway corridors. Environmental Management 13(3):365-370.