Kosteletzkya virginica (L.) C. Presl ex A. Gray var. aquilonia Fernald
Scientific Name: Kosteletzkya virginica (L.) C. Presl ex A. Gray var. aquilonia Fernald

| General Information | |
|---|---|
| Usda Symbol | KOVIA2 |
| Group | Dicot |
| Life Cycle | Perennial |
| Growth Habits | Forb/herbSubshrub, |
| Native Locations | KOVIA2 |
Plant Guide
Description
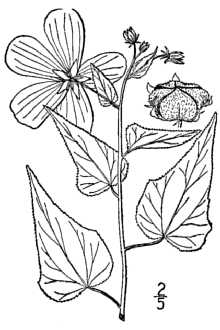
General: Virginia saltmarsh mallow is an herbaceous, flowering perennial which typically grows 3-5 ft tall and may spread as much as 4 ft across. Virginia saltmarsh does not spread vegetatively through rhizomatous growth, but rather occurs as an individual plant (Gould, 2000). Most plants usually do not reach the maximum height until near the end of the life cycle, characteristically after about 11 growing seasons (Moser et al., 2013 and LBJWC, 2015). Tiny stellate hairs making the plant somewhat rough to the touch cover both the stems and leaves. Leaves and stems are presented in a frequently branched and alternate pattern. The gray hued green leaves are triangular-ovately shaped and typically measure about twice as long as they do wide, ranging from 3-6 in (7-15 cm) long. The margins of the simple, stalked leaves vary within the species, but frequently range from coarsely to finely toothed. Saltmarsh mallow has a lengthy and striking bloom period, beginning as early as May and continuing into October. The showy flowers are reminiscent of Hibiscus flowers and range from a deep pink to a lightly pink hued white. The 5 petaled flowers are generally 1.5-3 in (4-7.5 cm) wide and have a short bloom period of 1 day, closing at night. The individual petals are rounded and measure about 1.5 in (4 cm) long. Numerous bright yellow stamens form a column around 5 fused carpels (Duncan, 1987). Exhibited below the 5 toothed summit of the stamen column are numerous anthers (Stone, 1973). Pollination of the flowers occurs by both insect-pollination and self-pollination (Ruan 2005). The fruit produced is a flat ring of 5 segmented capsules each containing 1 seed. The flattened shape of the fruit is a characteristic often used to distinguish Virginia saltmarsh mallow from Hibiscus. The hairy fruit capsules dry and turn from gray green to brown as the seed matures. The mature seed is irregularly shaped, smooth, and dark brown. Distribution: Virginia saltmarsh mallow is primarily a coastal plain species. The rosy pink blooms of Virginia saltmarsh mallow occur among the far more common grasses, rushes, and sedges of the marshes throughout the Mid-Atlantic region as far north as New York, south to Florida, and west to the coastal regions of east Texas (Godfrey, 1981). Shetler (2003) reports that Virginia saltmarsh mallow also occurs in Bermuda and the West Indies. Virginia saltmarsh mallow can be grown in USDA hardiness zones 5b-9 although it may not be consistently winter hardy in zone 5b (MBG, 2017). For current distribution, please consult the Plant Profile page for this species on the PLANTS Web site. Habitat: The National Wetland Plant List categorizes Virginia saltmarsh mallow as an obligate wetland plant for all wetland regions in which it occurs meaning it almost always occurs in wetlands (Lichvar, 2014). It commonly occurs on the coastal plains in tidal and non-tidal marshes, swamps, and along the edges of wetlands. It is salt tolerant and grows in brackish waters, but may occur in fresh water as well. It will grow in shallow standing water and also occurs in wet ditches (Ingersoll, Natural Resources Conservation Service Plant Guide Virginia saltmarsh mallow (Kosteletzkya virginica). Photo by Scott Snell, USDA-NRCS, Plant Materials Program. 2005). Other plant species associated with Virginia saltmarsh mallow varies with the salinity of the environment where it occurs. In brackish waters it is often associated with saltmeadow cordgrass (Spartina patens), saltgrass (Distichlis spicata), narrowleaf cattail (Typha angustifolia), smooth cordgrass (Spartina alterniflora), and big cordgrass (Spartina cynosuroides). Whereas in fresh water environments it is more often associated with crimson-eyed rose mallow (Hibiscus moscheutos), sweetscent (Pluchea odorata), common reed (Phragmites australis), and common threesquare (Schoenoplectus pungens) (Perry, 1997). It grows best in these habitats when the soils have a high sand content, but will tolerate some clay soils (LBJWC 2015). It also performs better in acidic soils and will do well in salt affected soils with a soil salinity range of 0.5-1.5% (Ruan et al. 2008). Virginia saltmarsh mallow acts as a non-dominant component in the varied plant communities that make up the diverse wetland ecosystems in which it occurs.
Adaptation
Kosteletzkya spp. are native to Malay Archipelago, Madagascar, Africa, southern Europe, Caribbean, North America, and South America. Virginia saltmarsh mallow is the only species of the genus with a natural range that covers a large area of North America (Shetler, 2003). Virginia saltmarsh mallow seedlings are salt tolerant and are naturally adapted to salt marshes and brackish environments where they thrive with regular inundation. At low salinity levels (5 gL-1) shoot and root growth are stimulated, but are progressively reduced as salinity levels increase. Roots exclude the uptake of sodium ions while enhancing the uptake of potassium ions to reduce the negative effects of saline waters. Additionally, sodium is directed to older leaves minimizing its effect on shoot growth (Blits and Gallagher, 1990 a,b). Although seedlings are salt tolerant, higher salinity levels negatively impact reproduction. Increased salinity levels negatively affect germination rates due to decreased seed permeability (Poljakoff-Mayber et al., 1994). Research has shown that seedlings recover and respond positively to extended periods of inundation through the formation of adventitious roots and increased root porosity (Zhou et al. 2012). It will grow well in fresh water environments as well, however does not compete well against most fresh water species. Similarly, it may also survive at less wet, more upland sites, however is often out competed by earlier germinating species (Ruan et al., 2008). Virginia saltmarsh mallow self-propagates and increases the range of dispersal by means of water transportation. The movement of tidal waters assists seed distribution via the ability of the seed to float due to air pockets (Poljakoff-Mayber et al., 1992).
Uses
Commercial crop: Virginia saltmarsh mallow has the potential to become a multiuse species as an agricultural crop on salt affected farmlands that may no longer be fit to produce traditional crops due to the negative impacts of soil salinization. Potential products produced from the plant could include biofuels, protein rich animal feed, animal bedding (cat litter), bioabsorbent, and hydromulch (Gallagher, 2015; Raun et al., 2008; Moser et al., 2013; Vaughn et al., 2013). The high oil content of Virginia saltmarsh mallow seeds is equivalent to that of soybeans, the primary plant source for biodiesel in the United States. Biodiesel produced from Virginia saltmarsh mallow seeds has been tested and found to be within ASTM D6751 and EN 14214 standards (Moser et al., 2013). Some research is looking at salt tolerant species such as Virginia saltmarsh mallow as a genetic source to genetically engineer improved salt tolerance of conventional crops (Wang et al., 2015). Cook et al. (1989) developed techniques for tissue culture and cloning of mutants that displayed valuable traits. Furthermore, Li and Gallagher (1996) demonstrated methods for the introduction of genes. An additional advantage of converting salt affected farmland to Virginia saltmarsh mallow production is that common agricultural equipment is highly compatible. Moreover, brackish water may be used to irrigate Virginia saltmarsh mallow allowing the producer to reserve freshwater irrigation for traditional agricultural crops (Moser et al., 2013). Conservation practices: Virginia saltmarsh mallow is a valuable buffer plant in areas between aquatic systems and farm production fields. It minimizes movement of particulates while capturing nutrients reducing the amount of nutrient runoff into surrounding waters (Gallagher, 2015). Studies have also shown that Virginia saltmarsh mallow has potential as a plant used for carbon sequestration. As a perennial with a large taproot system, use of the plant for long term, below ground carbon storage has merit (Halchak, 2011). Virginia saltmarsh mallow acts as a valuable nurse crop to assist the conversion of non-arable coastal farmland to valuable wetland by encouraging the recruitment of saltmeadow cordgrass and eastern baccharis (Baccharis halimifolia) (Voutsina et al., 2015). Ornamental/landscaping: In addition to the long flowering period providing great color, the tolerance of Virginia saltmarsh mallow to a variety of soils, salt spray, salt affected soils, and a wide range of moisture levels make it a valuable and versatile ornamental plant. It is not a common nursery ornamental, but is available from several specialty nurseries. Demand for it is likely to increase as the nursery industry promotes the benefits of the species as an ornamental. Additionally, it has been named wildflower of the year by multiple organizations (Haynes, 2004). Given the traits of Virginia saltmarsh mallow, it is also a valuable addition to rain gardens and pollinator gardens and is listed as a recommended species for both (Roos and Woodson et al.). Wildlife: Virginia saltmarsh mallow is valuable plant for a range of wildlife species. It provides value and is an attractant for butterflies and hummingbirds (LBJWC, 2015). It is a known larval host plant for painted lady butterflies (Vanessa cardui) (Hampton, 2009). The flowers of Virginia saltmarsh mallow provide an ample pollen and nectar source for a variety of insects and the ruby-throated hummingbird (Heiser, 2015; Ruan, 2008). The high oil content of Virginia saltmarsh mallow seeds make the mature fruit an energy rich and valuable food source for migratory birds and small mammals (Travis, 2014 and Ruan, 2008).
Ethnobotany
In an extensive study on medicinal plants used by indigenous tribes of the southeast, Kimberly Hutton (2010) described the use of Virginia saltmarsh mallow to treat multiple physical ailments. The Seminole tribe of Florida prepared the plant as a remedy to treat cramping, heat exhaustion, and to induce labor. Virginia saltmarsh mallow has multiple culinary applications. The edible flowers may be used as a colorful plate enhancement and eaten raw. Additionally, the flowers are also used to make a tea. Leaves are used as a potherb. The cooked root serves as a vegetable. Boiling any part of the plant produces an egg white substitute by extracting and concentrating the sugars and mucilages (Puckhaber, 2002). In China, an extract of the leaves is used to produce Virginia saltmarsh mallow wine (南京大学, 2016).
Status
Weedy or Invasive: Following a 10 year observation period of a large scale planting of Virginia saltmarsh mallow to an introduced environment, researchers concluded that it was not invasive due to the lack of rhizomatous spread. Following dormancy, new growth each year only occurs at the root crown of the plant. Furthermore, during the observation period native plant communities were not displaced (Ruan et al., 2008). Please consult with your local NRCS Field Office, Cooperative Extension Service office, state natural resource, or state agriculture department regarding its status and use. Please consult the PLANTS Web site (http://plants.usda.gov/) and your state’s Department of Natural Resources for this plant’s current status (e.g., threatened or endangered species, state noxious status, and wetland indicator values).
Planting Guidelines
Virginia saltmarsh mallow is a low maintenance perennial that is easy to grow. Propagation by seed is the most efficient means of reproduction. Soft wood cuttings are a possible means of propagation, but not recommended unless cloning is desired. Tip cuttings are more successful and root more readily than nonterminal cuttings (Haynes, 2004). Plugs produced in a greenhouse or more mature containerized plants should be used for restoration field plantings and installed in early spring. Production fields can be direct seeded in a well prepared seedbed following the threat of frost in mid-May to mid-June. Gallagher and Seliskar (2015) successfully demonstrated that production fields may also be established using no-till seeding equipment and methods. Seed should not be collected until the fruit has dried and turned brown indicating maturation of the seed. Disinfestation of the seed may be necessary before placing the seed in cold storage if the weevil population is significant (LBJWC, 2015). The seed germinates readily in wet, warm soils with full sun (Shetler, 2003).
Management
Production field establishment using plugs and direct seeding should both be done into a clean, well prepared seedbed in spring after the threat of the last frost. Successful direct seeding establishments have been planted from May to June in Delaware. Trials performed by the University of Delaware have shown pre-emergent herbicides useful to minimize weed pressure. Post emergent, grass specific herbicides were also found beneficial for weed control. Additionally, glyphosate may be applied to control winter and early spring weeds while the Virginia saltmarsh mallow plants are dormant (Gallagher, 2015). Fertilizer application is recommended to improve growth rate, flowering and fruit production. However, significantly lower germination rates and higher mortality has resulted from seed produced by fertilized plants (Abeli et al., 2017). Therefore, fertilizer applications should be determined by the goals and purpose of the planting. Irrigation during extended dry periods is recommended for seed and biomass production. A study at the Cape May Plant Materials Center (2015) found that regularly irrigated production plots produced over four times more seed and significantly taller plants. Seed is harvested in the fall after the bloom period. To maximize the percentage of mature, viable seed and avoid seed loss due to shattering, seed should be harvested when the upper most fruit on the plant is still green and the lowest fruit is beginning to shatter (Gallagher, 2015). Seed may be harvested by hand or mechanically by means of combine or flail vacuum. On the northern extent of its range, some winterkill may occur over especially cold winters. Mowing production fields in the fall following harvest to chop the remaining stems and produce a mulch will provide some protection for the exposed plant crowns and improve survival. Adding additional mulch will further insulate the plants and improve overwinter survival in colder regions (Gallagher, 2015).
Pests and Potential Problems
No significant problems with disease of Virginia saltmarsh mallow have been documented (MBG, 2017). Some insect damage has become a problem in production fields. Flea-beetle and scentless plant bug herbivory has become an issue in more mature production fields. Insecticide applications have been used as an effective means of control (Gallagher, 2015). If harvested for seed, weevil infestation could cause extensive damage and significantly reduce the quality of the seed produced. Several species of weevils prefer stored grains and seeds as their primary food source. If weevils are present in the seed harvest and it is not disinfested before storage, weevil damage may continue to degrade the quality of the seed (LBJWC, 2015).
Environmental Concerns
Concerns , Use soil moisture sensors to measure the soil moisture of Kosteletzkya virginica (L.) C. Presl ex A. Gray var. aquilonia Fernald.
Concerns
Virginia saltmarsh mallow is rare at the northern extent of its range in New York (Tiner, 2009). Loss of suitable habitat is the primary concern for this species (Shetler, 2003).
Seeds and Plant Production
Plant Production
Plant Production
Virginia saltmarsh mallow pollination occurs by means of both insect pollination and self-pollination. Self-pollination occurs through the use of stylar movements at the end of the flowering period if insect pollination has not occurred (Ruan, 2005). Seed yields of first year production fields have ranged from 569 lb/ac to 854 lb/ac (Ruan, 2008). Seed or softwood cuttings can be used for propagation. Mature seed germinates readily in moist to wet, warm (28°-30°C) soils following a period of soaking in warm water (LBJWC, 2015; Poljakoff-Mayber, 1994). Germination occurs quickly using fresh water, usually within 24 hours. Some seed dormancy may be displayed due to the thick seed coat causing a lack of permeability. Damage to the seed coat incurred naturally in the soil or via artificial stratification means increases seed permeability and germination rates (Poljakoff-Mayber, 1992). Germination may be achieved using brackish water, but success varies between different populations in response to salinity levels. A negative correlation between the rate of germination and salinity levels has been displayed. This trend is intensified in scarified seeds compared to untreated seeds (Gallagher, 2015; Poljakoff-Mayber, 1994). Softwood cuttings should be treated with a talc based IBA (Indole-3-butyric acid) rooting hormone. Both leafless and leaved cuttings are viable options for propagation. Terminal softwood cuttings provide the greatest rooting success, however nonterminal cuttings are also viable. All cuttings should be 3 in (8 cm) long. Terminal cuttings should have 2-3 leaves and nonterminal cuttings should have 2-3 nodes. Cuttings can be rooted in coarse perlite and kept wet during daylight hours using an intermittent misting system. Rooting should occur within several weeks. Cuttings should be closely monitored during this time and misting should be terminated when the cuttings have produced a self-sustaining root system (Haynes, 2004). Cultivars, Improved, and Selected Materials (and area of origin) These plant materials are somewhat available from commercial sources. Named cultivars of Virginia saltmarsh mallow include ‘Immaculate’ and ‘Ace Basin’. ‘Immaculate’ was selected for its large pure white flowers and is reported to have a later blooming period than wild collections (Raymond, 2015). ‘Ace Basin’ has consistent pink blooms. Additional, less common, named varieties are 'Cassandra Loos' and ‘Peter Loos’. 'Cassandra Loos' was selected for its larger overlapping pink petals and named after the late wife of Peter Loos, former president of The Native Plant Society of Texas. The ‘Peter Loos’ variety was selected for its darker pink petals (Grant, 2011).
Literature Cited
南京大学. 2016. Wine brewed by Kosteletzkya virginica leaf extract and other plant components and preparation method of wine. Patent Number: CN 103897947 A. Date issued: 5 October. Abeli, T. L., Brancaleoni, R. Marchesini, S. Orsenigo, G. Rossi1, and R. Gerdol. 2017. Fertiliser application positively affects plants performance but reduces seed viability in seashore mallow (Kosteletzkya pentacarpos): implication for biomass production and species conservation. Ann. Appl. Biol. 170(2):263-272. Blits, K.C. and J.L Gallagher. 1990. Salinity tolerance of Kosteletzkya virginica I. Shoot growth, ion and water relations. Plant, Cell and Environ. 13:409-418. Blits, K.C. and J.L Gallagher. 1990. Salinity tolerance of Kosteletzkya virginica II. Root growth, lipid content, ion and water relations. Plant, Cell and Environ. 13:419-425. Cook, D.A., D.M. Decker, and J.L. Gallagher. 1989. Regeneration of Kosteletzkya virginica (L.) Presl. seashore mallow from callus cultures. Plant, Cell, Tissue and Organ Culture 17:111-119. Duncan, W.H. and M.B. Duncan. 1987. The Smithsonian guide to seaside plants of the Gulf and Atlantic coasts. Smithsonian Institution Press. Washington, D.C. Gallagher, J.L. and D.M. Seliskar. 2015. Seashore mallow – The summer of 2015. Univ. of Delaware, Halophyte Biotechnology Center. Gallagher, J.L. and D.M. Seliskar. 2015. Provisional seashore mallow planting, growing, and harvesting protocol. [Online]. Available at http://extension.udel.edu/blog/tag/seashore-mallow/ (accessed 28 Sept. 2017). Univ. of Delaware, College of Agriculture and Natural Resources. Newark, DE. Godfrey, R.K. and J.W. Wooten. 1981. Aquatic and wetland plants of the southeastern United States: Dicotyledons. Univ. of Georgia Press. Athens, GA. Gould, L.L., Lofland L., and I.H. Stuckey. 2000. Coastal plants from Cape Cod to Cape Canaveral. Univ. of North Carolina Press. Chapel hill, NC. Grant, G. 2011. Greg Grant plant introductions. [Online]. Available at http://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=7&ved=0ahUKEwiW-_GC6tnWAhXGPiYKHcyVD30QFgg4MAY&url=http%3A%2F%2Fwww.texasroserustlers.com%2Fapp%2Fdownload%2F756161158%2FGreg%2BGrant%2BPlant%2BIntroductions.pdf&usg=AOvVaw2GIVMnCXPXB-eG2-MCq0X3 (accessed 05 Oct. 2017). Stephen F. Austin State Univ. Nacogdoches, TX. Halchak, J.L., D.M. Seliskar, and J.L. Gallagher. 2011. Root system architecture of Kosteletzkya pentacarpos (Malvaceae) and belowground environmental influences on root and aerial growth dynamics. Am. J. Bot. 98:163-174 Hampton, N. 2009. Lady Bird Johnson Wildflower Center. Available at https://www.wildflower.org/expert/show.php?id=4874 (accessed 27 Sept. 2017). Univ. of Texas. Austin, TX. Haynes, C.L. and W.R. Graves. 2004. Kosteletzkya virginica can be rooted from leafy or leafless stem cuttings. J. Environ. Hortic. 22(4):173–175. Heiser, C.A. 2015. Habitat gardening for wildlife. Virginia Cooperative Extension Master Gardener Program. Virginia State Univ., VA. Virginia Department of Game and Inland Fisheries, Habitat Partners Program. Henrico, VA. Hutton, K. 2010. A comparative study of the plants used for medicinal purposes by the Creek and Seminole tribes. Univ. of South Florida, Tampa, FL. Ingersoll, D.C. and S.L. Day. 2005. Propagation protocol for Virginia saltmarsh mallow. Native Plants J. 6.3:245-246. LBJWC (Lady Bird Johnson Wildflower Center). 2015. Kosteletzkya virginica. [Online]. Available at https://www.wildflower.org/plants/result.php?id_plant=KOVI (accessed 27 Sept. 2017). Lady Bird Johnson Wildflower Center, Univ. of Texas at Austin, Austin, TX. Li, X. and J.L Gallagher. 1996. Expression of foreign genes, GUS and hygromycin resistance in the halophyte Kosteletzkya virginica in response to bombardment with the Particle Inflow Gun. J. Exp. Bot. 47:1437-1447. Lichvar, R.W. 2014. The national wetland plant list: 2014 wetland ratings. Phytoneuron J. 41:1-42. MBG (Missouri Botanical Garden). Gardening Help – Kosteletzkya virginica. [Online]. Available at http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?kempercode=c709 (accessed 27 Sept. 2017). Missouri Botanical Garden, St. Louis, MO. Moser, B.R., B.S. Dien, D.M. Seliskar, and J.L. Gallagher. 2013. Seashore mallow (Kosteletzkya pentacarpos) as a salt-tolerant feedstock for production of biodiesel and ethanol. Renewable Energy 50:833-839. Perry, J.E. and R.B. Atkinson. 1997. Plant diversity along a salinity gradient of four marshes on the York and Pamunky rivers in Virginia. Department of Resource Management and Policy, Virginia Institute of Marine Science, College of William and Mary, Gloucester Point, Virginia. Poljakoff-Mayber A., G.F. Somers, E. Werker, and J.L. Gallagher. 1992. Seeds of Kosteletzkya virginica (Malvaceae): Their structure, germination, and salt tolerance. I. Seed structure and germination. Am. J. Bot. 79:249-256. Poljakoff-Mayber A., G.F. Somers, E. Werker, and J.L. Gallagher. 1994. Seeds of Kosteletzkya virginica (Malvaceae): Their structure, germination, and salt tolerance. II. Germination and salt tolerance. Am. J. Bot. 81(10):54-59. Puckhaber, L.S., R.D. Stipanovic, and G.A. Bost. 2002. Analyses for flavonoid aglycones in fresh and preserved Hibiscus flowers. 556–563. In: J. Janick and A. Whipkey (eds.), Trends in new crops and new uses. ASHS Press, Alexandria, VA. Raymond, L. 2015. Uncommon and astonishing plants. [Online]. Available at http://www.louistheplantgeek.com/a-gardening-journal/1199-kosteletzkya-virginica-080815 (accessed 05 Oct. 2017). Louis Loves Gardening, Hopkinton, RI. Roos, D. Native plants for bee forage for the North Carolina Piedmont. [Online]. Available at https://growingsmallfarms.ces.ncsu.edu/wp-content/uploads/2012/08/BeePlants2015.pdf?fwd=no (accessed 27 Sept. 2017). North Carolina Cooperative Extension, NC State Univ. and N.C. A&T State Univ. Ruan, C.J., H. Li, Y.Q. Guo, P. Qin, J.L. Gallagher, D.M. Seliskar, S. Lutts, and G. Mahy. 2008. Kosteletzkya virginica, an agroecoengineering halophytic species for alternative agricultural production in China's east coast: Ecological adaptation and benefits, seed yield, oil content, fatty acid and biodiesel properties. Ecol. Eng. 32(4):320-328. Ruan C. J., P. Qin, and Y. G. Xi. 2005. Floral traits and pollination modes in Kosteletzkya virginica (Malvaceae). Belgian J. Bot. 138(1): 39-46. Shetler, S. 2003. Virginia wildflower of the year brochure. [Online]. Available at http://vnps.org/wildflowers-of-the year/2004-seashore-mallow-kosteletzkya-virginica/ (accessed 27 Sept. 2017). Virginia Native Plant Society, Boyce, VA. Tiner, R.W. 2009. Field guide to tidal wetland plants of the northeastern United States and neighboring Canada. Univ. of Massachusetts Press. Amherst, MA. Travis, T. and S. Brown. 2014. Pocket guide to eastern wetlands. Stackpole Books. Mechanicsburg, PA. Vaughn, S.F., B.R. Moser, B.S. Diem, L.B. Iten, D.M. Seliskar, and J.L. Gallagher. 2013. Seashore mallow (Kosteletzkya pentacarpos) stems as a feedstock for biodegradable absorbents. Biomass and Bioenergy 59:300-305. Voutsina, N., D.M. Seliskar, and J.L. Gallagher. 2015. Kosteletzkya pentacarpos as an ecosystem engineer for transitioning coastal agricultural land to wetland during sea level rise. Estuaries and Coasts 38:35-44. Wang, H., X. Tang, H. Wang, and H. Shao. 2015. Physiological responses of Kosteletzkya virginica to coastal wetland soil. Sci. World J. (2015)354581. Woodson, D., T. LeRoy, L. Miller, M. Sturdivant, and B. Clayton. Texas rain garden plant list. [Online]. Available at http://rainwaterharvesting.tamu.edu/files/2011/05/Rain-Garden-Plant-List-11-02-09.pdf (accessed 27 Sept. 2017). Texas AgriLife Extension Service. Texas A&M Univ. College Station, TX. Zhou, J., A.G. Qi, Y.C. Zhang, S.W. Wan, and P. Qin. 2012. Adventitious root growth and relative physiological responses to waterlogging in the seedlings of seashore mallow ('Kosteletzkya virginica'), a biodiesel plant. Aust. J. Crop Sci. 6(1)73-80. Citation Snell, S.C. 2018. Plant Guide for Virginia saltmarsh mallow (Kosteletzkya virginica). USDA-Natural Resources Conservation Service, Cape May Plant Materials Center, Cape May, NJ. Published 06/2018 For more information about this and other plants, please contact your local NRCS field office or Conservation District at http://www.nrcs.usda.gov/ and visit the PLANTS Web site at http://plants.usda.gov/ or the Plant Materials Program Web site: http://plant-materials.nrcs.usda.gov. PLANTS is not responsible for the content or availability of other Web sites. The U.S. Department of Agriculture (USDA) prohibits discrimination against its customers, employees, and applicants for employment on the bases of race, color, national origin, age, disability, sex, gender identity, religion, reprisal, and where applicable, political beliefs, marital status, familial or parental status, sexual orientation, or all or part of an individual's income is derived from any public assistance program, or protected genetic information in employment or in any program or activity conducted or funded by the Department. (Not all prohibited bases will apply to all programs and/or employment activities.) If you wish to file an employment complaint, you must contact your agency's EEO Counselor (PDF) within 45 days of the date of the alleged discriminatory act, event, or in the case of a personnel action. Additional information can be found online at http://www.ascr.usda.gov/complaint_filing_file.html. If you wish to file a Civil Rights program complaint of discrimination, complete the USDA Program Discrimination Complaint Form (PDF), found online at http://www.ascr.usda.gov/complaint_filing_cust.html, or at any USDA office, or call (866) 632-9992 to request the form. You may also write a letter containing all of the information requested in the form. Send your completed complaint form or letter to us by mail at U.S. Department of Agriculture, Director, Office of Adjudication, 1400 Independence Avenue, S.W., Washington, D.C. 20250-9410, by fax (202) 690-7442 or email at program.intake@usda.gov. Individuals who are deaf, hard of hearing or have speech disabilities and you wish to file either an EEO or program complaint please contact USDA through the Federal Relay Service at (800) 877-8339 or (800) 845-6136 (in Spanish). Persons with disabilities who wish to file a program complaint, please see information above on how to contact us by mail directly or by email. If you require alternative means of communication for program information (e.g., Braille, large print, audiotape, etc.) please contact USDA's TARGET Center at (202) 720-2600 (voice and TDD). For any other information dealing with Supplemental Nutrition Assistance Program (SNAP) issues, persons should either contact the USDA SNAP Hotline Number at (800) 221-5689, which is also in Spanish or call the State Information/Hotline Numbers. For any other information not pertaining to civil rights, please refer to the listing of the USDA Agencies and Offices for specific agency information.