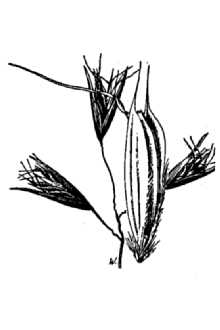
Danthonia californica Bol. var. americana (Scribn.) Hitchc.
Scientific Name: Danthonia californica Bol. var. americana (Scribn.) Hitchc.

| General Information | |
|---|---|
| Usda Symbol | DACAA |
| Group | Monocot |
| Life Cycle | Perennial |
| Growth Habits | Graminoid |
| Native Locations | DACAA |
Plant Guide
Alternate Names
Another common name is California danthonia. Synonyms include Danthonia americana and four botanical varieties of Danthonia californica: var. americana, var. californica, var. palousensis, and var. piperi.
Uses
Restoration and wildlife habitat: California oatgrass is an important native constituent of drier upland and moist lowland prairies as well as open woodlands. Therefore, it is commonly recommended for revegetation, wildlife plantings, and restoration of oak savannas, transitional wetlands, and grasslands, especially in the Pacific Coast states where it is most common. Native bunchgrasses like California oatgrass are valuable for enhancing biodiversity. Healthy stands can reduce invasion by exotic species yet exhibit a spatial distribution compatible with forbs (Maslovat 2001). Combined with other native grasses and forbs, California oatgrass improves habitat diversity for feeding, nesting, and hiding by songbirds (Oregon Department of Fish and Wildlife 2000), as well as other animals. The grains are eaten by small birds and mammals (Mohlenbrock 1992). Prairies with California oatgrass as a definitive species are also unique refuges for other endemic organisms. For example, the Ohlone tiger beetle (Cicindela ohlone) is an endangered (federally listed) predatory insect known only to five remnant stands of California native grassland in Santa Cruz County (Santa Cruz Public Libraries 2003). These rare grasslands, including the coastal terrace prairies, remain biodiversity “hotspots” and are considered in need of protection (Stromberg et al. 2001). Forage: As a rangeland plant, California oatgrass is well utilized by livestock and certain wildlife. Prior to maturity, the species is rated as good to very good forage for cattle and horses in the Pacific Coast states, but less palatable for sheep and goats. Ratings are lower for eastern, drier portions of its natural range (USDA Forest Service 1988). Others claim it is palatable to all classes of livestock and a mainstay grass for range grazing in places like Humboldt County, California (Cooper 1960). California oatgrass withstands heavy grazing (USDA Forest Service 1988, Cooper 1960). However, it is also reported that animals seek out and overgraze individual plants sometimes leading to rapid stand depletion (Crampton 1974). This species can provide green forage year round in some areas. Under moderate grazing it stools readily, forms a “sod” (ie. the bunches coalesce), and can produce a substantial volume of high quality forage. Less desirable species diminish as the sod forms. Higher nutritional content and grazing preference make California oatgrass desirable in a management system over soft chess (Bromus mollis) (Heady et al. 1963). Protein analysis is high at 8 to 26 percent; the low point coming in January after the herbage has been leached by precipitation (Cooper 1960). This species has formed stands dense enough for haying in California (USDA Forest Service 1988). Pollinators: California oatgrass is used as food by the caterpillar larva of certain butterflies including two skippers (Hesperia lindseyi and Hesperia columbia) (Robinson et al. 2007). It is an important component of native grasslands that form critical habitat for other butterflies including the vulnerable Vancouver ringlet (Choenonymphya tullia insulana), Taylor’s checkerspot (Euphydryas editha taylori) (Chappell 2006), and the endangered Fender’s blue butterfly (Icaricia icarioides fenderi) (Collins 2006). Cover and turf: Other potential uses include cover and erosion control in vineyards, young orchards (Edminster 2003), grassy lanes, and parks, as well as along trails. As a candidate for native lawn, this species can be planted and mowed to maintain a turf-like stand in landscape settings or elsewhere (Wrysinski 2004, Daniels 2007, Amme 2003). California oatgrass persists along compacted hiking trails and takes heavy foot traffic, trampling, and moderate summer moisture stress. It also has potential as a stay-green firebreak (Edminster 2003, Fire Safe Council 2007).
Status
Please consult the PLANTS Web site and your State Department of Natural Resources for this plant’s current status (e.g. threatened or endangered species, state noxious status, and wetland indicator values).
Description
California oatgrass (family: Poaceae) is a slow establishing yet long lived, cool season (C3) perennial bunchgrass of intermediate texture. Its stems (culms) grow 30-100 (10-130) cm tall and disarticulate (separate) at the lower nodes (joints). The leaf sheaths are smooth to densely hairy. Leaves are both basal and attached to the stem with the upper blades being 8-25 (10-30) cm long, flat to in-rolled, and spreading to abruptly bent. The ligule (at the throat of the leaf blade) is less than 1 mm and fringed with small straight hairs. Additionally, 1-3 mm long, soft spreading hairs appear at the leaf collar and throat (photo). Flowering occurs in May or June depending on location. The panicle (inflorescence) is 2-6 cm long, loose, and open with 1-5 (3-6) broadly spreading spikelets. Glumes are 14-18 mm long. There are 3-8 (5-10) florets (flowers) per spikelet (see photo). Lemmas are 5-10 (8-15) mm long, hairy along the margins with stiff awned teeth and an abruptly bent awn that is (4) 8-12 mm long (Darbyshire 2003, Hickman 1993, Hitchcock et al. 1969, Klinkenberg 2007). California oatgrass Reprinted with permission, University of Washington Press Leaf blades often bend at a broad angle from the stem and hairs fringe or replace the ligule (var. californica). .Additional tufts of hairs appear at the leaf collar. Photo by Steve Matson used with permission. Flowering and seed formation: California oatgrass produces seed from both open flowers that allow for cross-pollination and closed flowers that have obligate self-pollination. Its open pollinated flowers are referred to as chasmogamous and the seed they produce are chasmogenes (chasmogamic seed). Closed pollinated flowers are cleistogamous and the seed they produce are cleistogenes (cleistogamic seed). The chasmogamous seed is produced in the exposed panicle and is sometimes referred to as terminal seed. In contrast, the cleistogamous seed is primarily found at the lower nodes of the flowering culm and typically remains enclosed in the leaf sheaths (Dobrenz and Beetle 1966, Campbell et al. 1983). Spikelets are flattened with 5-10 flowers (florets) each. Photo by Steve Matson used with permission. There are differences between chasmogamic and cleistogamic seed production. Sometimes referred to as hidden seed, cleistogenes are typically shorter or longer (narrower) and larger than seed from the inflorescence and some lack a developed lemma and palea (Dobrenz and Beetle1966). Most commonly, there are 3-4 spikelets per flowering culm and 5-6 florets per spikelet. However, a single lower node can bear 6-7 cleistogenes and there can be 5-6 such nodes per culm. Therefore, more hidden seed can be produced than terminal seed. Production of cleistogenes increases under grazing pressure (Dobrenz and Beetle 1966). Seed coat characteristics may also differ between the two seed types as evidenced by contrasting germination responses to acid scarification reported by Laude (1949). Bruns (2005) found that seed set was lower in chasmogamous spikelets compared to cleistogamous spikelets and more maternal reproductive effort was put into cleistogamous seed production. However, chasmogamous seed had higher and quicker germination rates, as well as a higher rate of early seedling growth compared to cleistogamous progeny. In contrast, Weatherwax (1928) states there are no consistent differences in the two types of caryopses (naked seeds) and that both types germinate alike and seedling plants are alike in appearance and vigor through flowering. By July or August after the panicle has matured and some of the terminal seed has shattered (fallen from the plant), the remaining culm will disarticulate (separate) at the basal node (Darris pers. obs.). Dispersal of remaining cleistogenes is probably aided by this process (Dobrenz and Beetle 1966). The dry stems with enclosed seed may wrap around the feet or limbs of passing animals (Darris pers. obs.). Similar species: California oatgrass can be confused with timber oatgrass (Danthonia intermedia) which has a shorter stature, more erect, compact panicle branches, glumes that are longer than the flowers which are rarely visible, and hairless leaf sheaths. The panicles of poverty oatgrass (Danthonia spicata) and one-spike oatgrass (Danthonia unispicata) are also much narrower and therefore more spike-like, the latter having a single spikelet in the inflorescence (Stewart and Hebda 2000). The lemmas of California oatgrass are smooth along the back with hairs only at the margins while the lemmas of poverty oatgrass have hairs at both positions (Kozloff 2005). Distribution: California oatgrass occurs naturally from British Columbia to southern California, east to Montana and Saskatchewan and south through the Rocky Mountains to New Mexico and Arizona. A form is also found in Chile (Hitchcock et al. 1969). For current distribution, please consult the Plant Profile page for this species on the PLANTS Web site.
Adaptation
General: California oatgrass has relatively broad adaptation and is considered a stress-tolerator. It occurs on a wide array of soil types from excessively drained sandy loams to less permeable silts and clays and from relatively infertile sites to rich bottomland. While the species occurs on more xeric sites such as sunny south and west slopes, adequate subsurface moisture or winter rains in milder climates are common themes. In California, arid sites do not support it; including the inner foothills of the Coast Range and Sierra Nevada foothills (Crampton 1974). Elevation range for the species is 0-2200 m (7200 ft) (Hickman 1993). Annual precipitation varies from 43 to 200 cm (17 to 79 in) in its natural range of habitats for the West Coast states. California oatgrass is also found on serpentine soils of Oregon and California, suggesting that at least some populations or races are especially tolerant to drought, calcium and other key nutrient deficiencies, high magnesium, and high levels of certain heavy metals such as nickel. Serpentine soils are often shallow and rocky with low levels of silts and clays (Brady et al. 2005). Based on soil types and site descriptions, California oatgrass appears to be adapted to moderately acid to alkaline soils (estimated pH 5.5-8). Depending on region, the species is classified as a facultative upland, facultative, and facultative wetland plant. Fertility requirement is considered low as is salinity tolerance (USDA NRCS 2007). However, its common occurrence on coastal prairies and bluffs suggests some local resistance to salt spray. California oatgrass is rated low for drought resistance (8 on a scale of 1-10), relatively high for fire resistance (3), low for shade tolerance (8), high for wildlife value (2), and relatively low for deer resistance (7) (Fire Safe Council 2007). In contrast, others rate drought tolerance as medium or moderate (USDA NRCS 2007, Wrysinski 2004) and the species is known to inhabit sites that are very dry in summer. Klinkenberg (2007) reports a soil moisture regime of 2.1 [0=xeric, 4=mesic, 8=hydric]. Maslovat (2002) describes California oatgrass as having high tolerance to fire along with other characteristics making it a good candidate for disturbance prone ecosystems. Habitat and plant communities: California oatgrass is a minor to dominant constituent of numerous woodland, shrubland, grassland, and transitional wetland habitats, particularly in the Pacific Coast States. Included among the woodland sites are several Garry oak (Quercus garryana) communities in California, Oregon, Washington, and southwestern British Columbia (Franklin and Dyrness 1973). This species is also an understory grass in certain lodgepole pine (Pinus contorta) communities in the interior Northwest as well as Jeffrey pine (Pinus jeffreyi) grass woodlands on serpentine soils of the Siskiyou Mountains region (Northwest
Habitat
Institute 2008, Franklin and Dyrness 1973). California oatgrass is prominent within a number of primarily graminoid communities. Significant populations occur west of the Cascades in Oregon and Washington on prairie and oak savanna habitat. Most of the annual precipitation is from 43-140 cm (17-55 in) (Wildlife Habitat Institute 2008). In the eastern foothills of the Oregon Coast Range where winters are cool and relatively moist but summers hot and droughty, the Danthonia californica Valley association occupies xeric, rocky, south and west facing slopes. The species represents up to 70 percent of the cover on these sites (NatureServe 2008). California oatgrass is also a dominant, co-dominant, or frequent component in grassy bald associations such as Danthonia californica- Eriophyllum lanatum and Festuca roemeri-Plectritis congesta. One or both of these associations occur in patches from the Georgia Basin of Southwest British Columbia south through the San Juan Islands, Puget Sound, western Columbia Gorge, and the Willamette Valley of Oregon. These sites are seasonally moist to very dry with shallow soils, rock outcrops, and moderate to steep slopes with southern to western exposures and an annual precipitation range of 74 to 185 cm (29 to73 in). California oatgrass is part of the sole remaining native-dominated prairie community of the south Puget Sound: the Festuca roemeri-Sericocarpus rigidus association. The habitat is moderately dry with relatively rich, deep, excessively drained, gravelly sandy loam soils (Chappell 2006). In maritime regions along the northern California Coast and into Oregon, winters are mild and wet and summers cool and dry. Here California oatgrass is a dominant, often signature plant of the coastal grasslands including three associations: Danthonia californica-Festuca rubra, Danthonia californica-Aira caryophyllea, and Deschampsia caespitosa-Danthonia californica (coastal). The extent of all these West Coast herbaceous communities was probably much greater prior to fire suppression, land use conversions, exotic weed invasion, and brush encroachment (NatureServe 2008). In this same area are California coastal scrub communities containing California oatgrass including the Baccharis pilularis-Danthonia californica association (NatureServe 2008). The species is also found in Ceonothus-Manzanita (Arctostaphylos spp.) shrublands or chaparral that extend from southwest Oregon throughout much of California. In this region the climate is typically very warm and relatively dry with about 43 to 76 cm (17 to 30 in) of annual precipitation. Soils are generally shallow over bedrock or are formed from coarse alluvial deposits and some are serpentine (Wildlife Habitat Institute 2008). California oatgrass also occupies transitional wetlands in western Oregon and western Washington. The Deschampsia caespitosa-Danthonia californica association is found in nutrient rich wetlands and flat bottomlands that are temporarily to seasonally flooded. Dry, hot summers and mild, wet winters are the norm. This and similar communities were historically maintained by frequent burning. Soils are usually fine textured silts and clays, moderately permeable to impermeable, with a high winter and spring water table (NatureServe 2008). An example of this community is French Flat in Josephine County, Oregon, where tufted hairgrass (Deschampsia caespitosa) dominates the seasonally hydric soils while California oatgrass dominates the mesic sites (Mousseaux 2004).
Establishment
Establishment of California oatgrass from seed can be problematic as the result of delayed germination and variable seed dormancy, as well as moderately slow seedling development combined with early competition from other species. Difficulties with reseeding Danthonia species were reported in the early to mid 1900’s when interest grew in reintroducing native perennial grasses on depleted or weedy grazing lands in California (Laude 1949). Seed dormancy and germination: Evidence suggests the variable seed dormancy in California oatgrass is the result of either single or double (combined) dormancy. The dormancy may be variants of seed coat imposed dormancy, physiological (embryo) or both. Moreover, the amount and possibly the type of dormancy can vary among populations of California oatgrass (Trask and Pyke 1998). It may also depend on crop year or seed lot (Laude 1949) within the same population because climatic conditions during seed development can influence the expression of seed dormancy. Furthermore, the length of time and conditions during storage may affect seed dormancy of grasses like California oatgrass by influencing after ripening (Simpson 1990). Amme (2008) reports that fresh seed or seed that is sown and watered immediately after collection can germinate readily at a high percentage. In contrast, germination is delayed after a period storage, suggesting that dormancy can deepen over time. Field observations indicate dormancy will also vary among seeds within the same seed lot of California oatgrass. In some years at Corvallis, Oregon, both fall seeding of fields and fall sowing of containers resulted in a portion of seedlings emerging within three weeks, while additional seedlings emerged as early as March in each of the following two springs (Darris per. obs.). Others report more continuous but prolonged germination and emergence periods. Some variability in the dormancy and germination in mechanically harvested lots may be due in part to aggressive seed combining and conditioning/cleaning processes. In these instances some dehulling (ie. hulling or separating the caryopsis or kernel from the lemma and palea), may occur and the seed coat (pericarp) is inadvertently scarified or nicked on some seeds and not others. Given the variable dormancy in California oatgrass seed, many methods have been used for improving germination with varying success. No treatment may be needed (Emery 1988, Dyer 2001) and direct sowing with untreated dry seed resulted in 60% germination in 21 days for Young (2001). Mechanical abrasion or “injury” with coarse sand paper or brush machines in the process of dehulling, acid scarification, or puncturing of the seed coat alone have improved germination by reducing seed coat imposed dormancy. However, mechanical scarification can be difficult to achieve without unwanted damage to the seed. In a study done by the Corvallis Plant Materials Center (PMC) (unpublished data) germination was greatly reduced by using a huller-scarifier (brush machine) to dehull and simultaneously scarify seed alone or in combination with prechilling (cold moist stratification) treatments. The mechanical procedure was far too aggressive and caused excessive damage to the seed and therefore significantly lower germination. This supports Laude (1949) who indicated mechanical scarification to weaken the seed coat did not appear feasible due to the protruding embryo being in an exposed position resulting in embryo injury. An alternative physical means of scarification may be the use of an oat huller to condition the seed. It appears to dehull more gently and reduce breakage to the ends of the seed compared to a huller-scarifier (Darris pers. obs.). Further testing is needed to confirm if this machine can simultaneously dehull and effectively scarify the seed coat to get good germination without resorting to acid treatment. In one greenhouse study, inflorescence (terminal) seed treated for 15 minutes with concentrated sulfuric acid (sp. gr. 1.84) as a means of scarification resulted in faster and higher seedling emergence for all 16 populations after 4 weeks, and for all but 4 populations after 16 weeks (Laude 1949). The acid both dehulled the seed and etched the seed coat. However, 15 minutes seemed too severe for cleistogenes. For terminal seed, 15 minutes of acid treatment gave the best results in the field (8-20% seedling emergence), 30 to 45 minutes gave the best results in a germinator (81% germination), and 20 minutes resulted in the highest emergence (19-27%) in a greenhouse. In contrast, 5 to 10 minutes gave the best results for cleistogenes in the field (4% emergence) and greenhouse (10% emergence), while 10 to 15 minute treatments were best in the germinator (71-81% germination). In all cases, performance declined for acid treatments beyond these time frames. This work underscores the fact that different results can occur between controlled environments and field conditions and between the two seed types of California oatgrass. It also demonstrates the importance of recommending the best treatments found in field tests for field use (Laude 1949). It would appear dormancy reduction in these trials was primarily the result of injury to or weakening of the seed coat and not dehulling (Laude 1949). Glumes or the seed covering formed by lemma and palea (the seed “hull”) can create seed dormancy in grasses (Simpson 1990) apart from the coat itself. However, whether in a germinator, greenhouse, or field and regardless of seed type, both untreated seed with lemma and palea intact and dehulled seed barely germinated while dehulled seed nicked with a scalpel showed a substantial improvement in percent germination and emergence. The author considers nicking the seed and acid treatment to be akin for the necessary purpose of weakening the seed coat. The seed coat imposed dormancy in California oatgrass may or may not be a form of physical dormancy (seed coat constraint on moisture imbibition). Laude (1949) found that dehulled but unscarified seed and acid scarified seed both adsorbed moisture similarly. As a result, the author suggests the dormancy is caused by restricted gas exchange or mechanical constraints and not the prevention of moisture uptake. However, based on structural characteristics of grass seed coats, Simpson (1990) argues that rate or specific location of moisture uptake by the seed rather than gas exchange can be explanations. This suggests the possibility of California oatgrass seed possessing a variant of “physical” dormancy but further investigation is needed. For reducing physiological dormancy, a solution of potassium nitrate (KNO3) or moist prechilling (cold moist stratification) alone have improved germination, as has giberellic acid (GA3) in combination with seed scarification, KNO3 plus scarification, or prechilling. Improvement with a combination of chemical and physical treatments supports the notion that California oatgrass can have complex or combined dormancy. Dobrenz and Beetle (1966) found that for both chasmogamic and cleistogamic seed, germination was similar and did not occur without blotters being soaked with KNO3. Results were 0% germination for controls and 10% germination using a solution of 0.2 % KNO3. In a study with four populations having 0 to 91% initial dormancy, a combination of seed scarification (by dehulling and rubbing seeds/caryopses between course sandpaper) and GA3 (300 ppm or 0.03%) improved cumulative germination to over 80% in all but one seed lot, while breaking over 90% of the dormancy. KNO3 only enhanced germination in combination with scarification and GA3 (Task and Pyke 1998). In a pilot study to enhance germination Trask (1996) found GA3 (400 ppm) alone to be the most successful treatment regardless of whether the seed was scarified or not. A combination of light, a 20/25°C (68/77°F) night/day temperature regime, and seven week prechill is suggested by Chirco and Turner (1986-2007). Unpublished work by the Corvallis PMC on two populations of California oatgrass demonstrated that moist prechilling of both inflorescence seed and cleistogenes at 5°C (39°F) for 45 to 90 days was effective in significantly improving germination as recorded for the first 28 days in a germinator. Other practitioners indicate improved germination with 30 days of moist prechilling at 3-4°C (37-39°F) in combination with manual removal of the hull (Keeley 2000), 12 to 13 weeks of cold moist stratification alone, or simple fall sowing (Boyer 2007a). From personal communication with Jebb (1995), Rose et al. (1998) report California oatgrass does better with a one to three day soak in running water followed by three months of cold moist stratification at 1-5°C (34-41°F). Guerrant and Raven (1995) achieved good germination with cold stratification at 5°C (41°F) for six weeks followed by warm stratification for six weeks using 16 hour days at 20°C (68°F) and 8 hour nights at 10°C (50°F). Knapp and Rice (1994) found seed viability and germination varied among populations. They had “high” germination rates by squeezing the caryopsis out from the glumes and lemmas, pre-treating with 400 ppm GA3, and cold moist stratifying for 2-3 weeks at 4°C (39°F). In successful field applications using fall sown seed, it may not be clear whether germination was enhanced by cold moist stratification over winter, or weakening of the seed coat by soil influences, or both. Finally, Maslovat (2001) reported that California oatgrass required light to germinate and associates this trait with natural regeneration after disturbance. However, for the Corvallis PMC some seed germinated in the dark, suggesting light may not always be necessary. Summary: California oatgrass seed can be nondormant, for example when “fresh”, or commonly possess one or more types of dormancy that need to be overcome for germination to occur. The kind or amount of treatment(s) required, if any, may be specific to population, crop year, seed lot, seed type, storage conditions, or age of seed. The Association of Official Seed Analysts (AOSA) has not set official rules for testing the germination of California oatgrass. Nevertheless, for many seed lots the most practical method to improve germination without resorting to chemical enhancements is cold moist stratification. This can be done by fall seeding or moist prechilling in a controlled environment for 21-120 days. For other seed lots, dehulling/scarification of the seed will greatly improve germination if the seed coat can be scratched, nicked, or eroded without undue injury to the embryo. Manual methods to remove the caryopsis from the hull (squeezing out the caryopsis, rolling seed between rubber mats) along with the use of sandpaper or scalpels to weaken the surface are not practical on a large scale. A mechanical means is needed for large seed lots but a brush machine appears to be too aggressive. Gentler mechanical means of dehulling and scarifying, such as the use of an oat huller, needs further evaluation. Acid scarification requires special safety precautions but merits consideration. Finally, in some cases a combination of both stratification and scarification may prove to be the most effective way of reducing dormancy. Natural establishment: Maslovat (2001) examined and described factors influencing natural and assisted establishment of California oatgrass in Garry oak ecosystems of Southwest British Columbia. Characteristics of this grass suggest it is an important colonizer following disturbances such as fire. While a modest seed producer, its seed dormancy and need for light to germinate help create a persistent seed bank (Maslovat 2001). The seed often remains viable in the ground for years. Stands can be resurrected from this latent seed by mowing or other disturbances (Amme 2003). Seedling recruitment appears aided by retention of shallow litter or moss and variable microtopography, especially minor depressions and grooves that favor higher moisture storage. Deeper litter, often exacerbated by long term fire suppression, may act as an impediment to root penetration and seedling emergence. However, raking of the soil to completely remove litter and clippings can reduce seed germination and establishment on some sites (Maslovat 2001). The awns found on unprocessed seeds of California oatgrass presumably improve dispersal by attaching to passing animals. In addition, the same awns are hygroscopic (bend and straighten with wetting and drying), a trait implicated in natural seed burial. They may also assist in seed migration and selection of more favorable microsites (Maslovat 2001). Site preparation: Keys to establishing California oatgrass for revegetation and other goals are preplant weed control and proper seedbed preparation. Starting situations can vary greatly, calling for site specific strategies. Among the most difficult cases are abandoned fields and other areas dominated by exotic weeds that have an extensive weed seed bank built up in the soil. One option for site preparation is to fallow the area for one to three years with repeated tillage operations following each flush of new weeds in order to reduce the weed seed bank prior to sowing. A nonselective herbicide (usually glyphosate) can be used in combination with the tillage (Darris 2003, Campbell 2004, Stromberg et al. 2002, Stromberg and Kephart 2003). Others suggest the weed seeds are usually too numerous and better addressed with minimal soil disturbance and two years of herbicide application followed by planting with a no-till drill (Boyer 2007b). In restoring oak savanna or other grassland habitats, plowing or other major tillage operations are not recommended next to existing desirable native trees and shrubs, on sites that already have some native plant diversity or rare plants, or before ground nesting birds have completed their nesting cycle (Campbell 2004). In order to preserve native perennial grasses already on site, herbicides are a poor choice where weedy annual grasses are a problem because (except for fine fescues) the chemicals used to control one group also control the other (McClaran 1981). Such sites or inclusions are better candidates for interseeding, no-till drilling, or transplanting. Besides tillage and herbicides, other site preparation methods include burning, grazing, mowing, soil solarization or combinations thereof (Campbell 2004, Rodgers 1981, Kephart and Amme 1992). Where permitted, burning can be a good choice for site preparation depending on existing conditions (Campbell 2004, Rogers 1981). Seed testing: Given the potential for highly variable seed dormancy, it is strongly recommended that all seed lots of California oatgrass be given a TZ (tetrazolium chloride) test to determine total viability along with a germination test before purchasing and planting. The difference between the two tests will give an estimate of the percent dormant seed. If dormancy is low, special stratification or scarification treatments are unnecessary. If dormancy is high, pure live seeding rate calculations need to be based on total seed viability and not percent germination. Direct seeding: Direct sowing of California oatgrass in the Pacific Northwest USA is usually best in late summer or fall (August-October) to naturally stratify the seed over winter (Maslovat 2001), assuming high dormancy in the seed lot. However, seeding date may be extended into the early winter (November) if site conditions have low risk of disturbance after seeding and the time outdoors remains long enough for natural stratification to occur (cool moist conditions may be required for up to 4 months). Emergence in milder winter growing climes typically occurs in late February and March (Boyer 2007b). Late winter or spring sowing with dormant seed can also result in germination the following March (Maslovat 2001), but seed losses from predation, erosion, weed competition, natural mortality, or other factors are potentially greater due to the lengthier period of inactivity. Spring planting may work equally well in some regions if seed is primarily nondormant or cold moist stratified or scarified in advance. On occasion, de-awing of California oatgrass seed may be needed to facilitate movement through certain seeding machinery. However, Maslovat (2001) describes the ecological importance of the awn and states “restoration of this species will only be successful if the disaspores [seeds] remain awned”. Despite this pronouncement, germination of some seed lots benefit from dehulling/scarifying and substantial awn removal cannot be prevented during mechanical or acid conditioning. The most successful stands of California oatgrass are usually achieved by drilling, no-till drilling, or broadcasting the seed alone rather than in a mix. As with natural establishment, very shallow soil coverage (0.6 cm or 0.25 in or less) is critical because of the light requirement. Broadcast seeding may additionally benefit from irregular surfaces. Mixing the species with nondormant seed of fast establishing grasses or forbs, native or otherwise, can lead to poor establishment because quicker germinating species will occupy the space first. To achieve a more natural appearance in prairie restoration, California oatgrass can be sown alone in irregular patches within more favorable soil inclusions then surrounded by a variety of higher diversity plantings. This is similar to the mosaic seeding approach described by Campbell (2004). Plant diversity can also be achieved afterwards by over seeding new stands the following spring or fall with forbs and/or other native grasses that have nondormant seed. Some restoration practitioners suggest seeding forbs a year before the grasses for improved species richness, as native forbs are more able to establish without grass competition (Clark and Wilson 2005). However, California oatgrass may be an exception and could be sown with forbs because of its seed dormancy. For California oatgrass to be sown in a mixture, the most viable options may be (1) combining it with species that have similar seed dormancy, (2) using it with low rates of a less competitive, more diminutive species or short-lived plants useful for winter cover, or (3) both. Species for the first option could include Columbia needlegrass (Achnatherum nelsonii) or Lemmon’s needlegrass (Achnatherum lemmonii). Choices for the second option include slender hairgrass (Deschampsia elongata) (Boyer 2007b, Darris 2003) which only lives 1 to 3 years, or better yet, annual hairgrass (Deschampsia danthonioides). However, both native hairgrasses establish readily when fall sown and should be limited to 0.5 kg/ha (~1/2 lb/ac) in a mix with California oatgrass. They are also prolific re-seeders. It is reported that California oatgrass has anywhere from 198,000 to 363,000 seeds/kg (90,000-165,000 seeds/lb) (Wrysinski 2004, Heritage Seedlings 2007, Darris and Lambert 2000, Guerrant and Raven 1995). The number probably depends on the degree of physical seed conditioning, genetics, and growing conditions at the time of seed formation. Dehulled and de-awned seed lots will be in the high end of the range. Each 1 kg of pure live seed (PLS) sown per ha will result in 20-37 live seeds/m2 (1 lb PLS/ac results in about 2-4 live seeds/ft2). Sown alone, suggested seeding rates for drilling are 10-16 PLS kg/ha (9-15 PLS lbs/ac), depending on goals and site conditions. Rates are high because of unpredictability and should be doubled for broadcast seeding. Amendments: A starter fertilizer is usually not recommended for slow establishing native grasses like California oatgrass as it encourages excessive weed competition. A covering of mulch such as a thin layer of native straw, hydromulch, or erosion blanket is particularly useful on steeper banks. For improved stability, the straw can be crimped into the soil or covered with a netting (Kephart and Amme 1992) such as jute. Weed control: Guidelines for the use of a number of herbicides for controlling weeds in native grass plantings in California are outlined by Drewitz and Anderson (2003). Pesticide labels vary by state and change over time, so the most current, local information must be reviewed and followed. Seed dormancy in California oatgrass can be put to good use. Sowing monotypic stands allows for fall and early winter germinating weeds to be controlled with glyphosate or other nonselective herbicide before the oatgrass seedlings emerge (Boyer 2007b, Darris 2003). After emergence and early growth of the California oatgrass, broadleaf weeds can further be controlled with a selective broadleaf herbicide applied at the right stage (Peachy et al. 2007). Mowing over the top of the oatgrass seedlings is a good alternative to control taller weeds, as is wicking with an herbicide, especially before the weeds go to seed. Timely, controlled grazing may also be beneficial for weed control in newer stands. Transplanting: Some practitioners have had more establishment success using transplanted seedlings instead of seed (Suttle and Thornsen 2007, Buisson et al. 2004, Angelo 2005, McClaran 1981). Reasons can include low germination rates and slow seedling and plant development the first year limiting the species ability to compete with weeds and other plants. Container grown seedlings are well suited to smaller projects. Initial costs are higher per acre, but site preparation requirements can be less and establishment risks lower compared to direct seeding. If a “sod” appearance is desired, a grid spacing of 12-15 cm (5-6 in) may be needed. Amme (2003) suggests a good “turf” of California oatgrass can be established with a spacing of 20-25 cm (8-10 in).
Management
As with all species, best management practices for California oatgrass can vary widely depending on the purpose of the planting or field (erosion control, turf, range, wildlife, or habitat restoration), available resources, site conditions, climate, stand composition, and other factors. Swards of native perennial grasses or grass-forb meadows containing California oatgrass can be improved and maintained with properly timed mowing, grazing, burning, herbicide applications, or combinations thereof. In California, the Pacific Northwest, and possibly other regions, doing nothing is often not an option in the long run because of ongoing and sometimes increasing competition from weedy annuals or invasive perennials, and the spread of shrubs or trees previously controlled or excluded by fire (Stromberg and Kephart 2003) . Mowing: Mowing is a viable option for controlling certain annual and perennial weeds as well as undesirable woody plants. For example, invasive perennial grasses such as tall oatgrass (Arrhenatherum elatius) are replacing native species on grasslands targeted for habitat conservation. From experimental work in western Oregon, Wilson and Clark (2001) report that after several years of late spring mowing at a 15 cm (6 in) height, both flowering and abundance of California oatgrass increased as a result of release from suppression by tall oatgrass. The annual mowing was timed to the flowering of tall oatgrass and its maximum above ground allocation. For annual weed control, mowing two to three times, especially the first year after establishment can be beneficial and may be required. In California, close mowing in the early spring (March) generally favors perennial grass establishment and enhances vigor. At the same time it reduces direct competition from weedy annuals and the production of annual grass seed along with their buildup in the soil bank (Kephart and Amme 1992). Annuals should be mowed to about 10 cm (4 in) in height after food reserves have been moved into their seedheads but before the large seeds are viable (Stromberg and Kephart 2003). A fall mowing also improves perennial grass growth while providing space and light for new seedlings (Kephart and Amme 1992) When used in a home garden, grass alley, trail side, or turf setting, California oatgrass can be mowed as low as 6-8 cm (2.5-3 in) in height (Darris pers. obs.). Daniels (2007) suggests mowing only once a year in early spring. The species will maintain itself as a tough, persistent “sod” of intermediate texture if plants are spaced tightly enough (Darris pers. obs.). It can be kept green year round if occasionally irrigated and cut back (Amme 2003). Grazing: Adaptive and flexible grazing techniques are an option for improving abundance of native grasses like California oatgrass and other desirable herbaceous plants in grassland communities (Stromberg and Kephart 2003, Menke 1992, Bartolome et al. 2004). However, universal prescriptions cannot be made due to variable site, timing, climatic, stand composition, and other factors. The amount of cover for this species can increase, decrease or remain unchanged under grazing. In California’s Coast Range grasslands, Bartolome and others (2004) report that California oatgrass had little response over time to seasonal grazing but increased when grazing was removed. In contrast, its foliar cover increased under continuous grazing and decreased when grazing ceased on California coastal grassland (Hatch et. al. 1999). Similarly, others observed higher cover (Hayes and Holl 2003) and increased vegetative growth and fewer competing annuals under moderate or even heavy grazing (Heady et. al 1963). California oatgrass and other native perennial grasses increased, range condition and health of the herd improved, and annuals decreased when heavy grazing was replaced by moderate stocking rates and deferred-rotation grazing (Cooper 1960). The species will develop a shorter more spreading form in response to clipping that can make it less noticeable to grazers (Edwards 1992). However, grazing (and burning) practices favorable to one native grass species may damage others (Hatch et. al. 1999). According to Menke (1992), grazing can be the primary step in a perennial native grass restoration project as well as ongoing maintenance. For restoring California native grasslands, he prescribes several days of high intensity, short-duration sheep or cattle grazing in order to remove the inflorescences of alien annual grasses before they set seed. The grazing event must be planned so that it still allows enough time for native perennial grasses (like California oatgrass) to flower and produce seed before spring soil moisture is exhausted. This action promotes increased vigor and crown cover of the natives. Secondarily, an intense period of heavy livestock grazing during midsummer dormancy of the perennial bunchgrasses reduces dead stems, litter buildup, and self-shading while hoof action enhances nutrient cycling by putting dead material in contact with the soil. Prescribed fire: Controlled burning is widely recognized as an important tool to control invasion of native and exotic woody plants in order to maintain prairies, ranges or other natural systems containing California oatgrass. As a species which evolved in western prairie ecosystems where fire is a natural process, it is generally tolerant to late summer burning. In a study by Hatch and others (1999) the species was unaffected by fire. However, it is less tolerant to fire than Nassella pulchra in California grasslands (Bainbridge and D’Antonio 2003). In these areas, fire is used to decrease the abundance of non-native species and increase or restore native vegetation but results are inconsistent (Bainbridge and D’Antonio 2003). Menke (1992) considers late spring-early summer burning to reduce alien weed seed production a viable enhancement tool for California grasslands with significant native bunchgrass populations. Burning is timed to the period when most weed seeds are still within the flower heads (panicles) so they can be destroyed. If litter levels are excessive, high mortality may result unless this fuel load is reduced in advance by mowing or grazing. Once stands are improved, burning is recommended only once every three to four years. Three years is about the time for alien annuals to recover to pre-fire levels (Menke 1992). Herbicides: Certain invasive non-native species that pose a continuing threat to native plant communities and other systems with California oatgrass can be effectively managed with herbicides. Whenever possible, they should be integrated with other management measures. Weed control for natural areas is described in depth by Tu and others (2001). References such as the Pacific Northwest Weed Management Handbook (Peachy et al. 2007) cover herbicide recommendations for cropping systems, non-crop areas, and other situations that can apply to California oatgrass.
Pests and Potential Problems
Few pest problems for California oatgrass have been recorded. However, it is one of many native grass hosts for the fungus (Gloeotinia temulenta) which causes blind seed disease, a potentially serious pest in ryegrass (Lolium spp.) seed production fields (Fischer 1944, Alderman 2001). Field burning is among several effective controls. The species can be infected by a nematode (Cynipanguina danthoniae) that causes leaf galls (Maggenti et al. 1974). Amme (1986) indicated that California oatgrass, along with other native grasses tested, appeared disease resistant during germination, transplanting, and growth in liners. No losses were attributed to damping-off. Rust (Puccinia sp.) and other stem or leaf diseases have not been regularly observed or have been of little consequence for seed producers (Kanegy 2007) and the Plant Materials Center, Corvallis, Oregon (Darris pers. obs.).
Environmental Concerns
Concerns , Use soil moisture sensors to measure the soil moisture of Danthonia californica Bol. var. americana (Scribn.) Hitchc..
Concerns
California oatgrass is not considered to be weedy within its natural range and is easy to control by mechanical or chemical means. However, because of seed dormancy, a resilient seed coat, and latent seed in the soil, individuals may continue to sporadically emerge several years after a stand is removed. The species is not reported to have toxic properties for domestic livestock, wildlife, or humans.
Seed and Plant Production
Plant Production
Plant Production
As with sowing California oatgrass for revegetation and other uses, planting new fields for agronomic seed increase and producing container nursery stock from seed may be confounded by poor or delayed germination due to seed dormancy. If dormancy is known or suspected, seed should have a TZ test to determine viability and then fall (Sept-Oct) sown or cold, moist stratified for 30-120 days. Dehulling or scarification may also be needed as described earlier. Seed production: Suggested seeding rates are high (11-20 PLS kg/ha or 10-18 PLS lbs/ac) to insure adequate stands since not all seed dormancy may be overcome. Clean, firm, weed free seedbeds and a seeding depth of 0.3 to 0.6 cm (0.13 to 0.25 in) are ideal. The suggested row spacing is 30-45 cm (12-18 in) but wider rows may be needed for cultivators or shielded row sprayers used for applying herbicides. To fit their irrigation systems and equipment, some growers produce the seed in nursery beds usually comprised of four narrow rows with wider (91-107 cm or 36-42 in) rows between the beds (Anderson 2008). Given issues of seed dormancy and slow establishment, a more reliable alternative is to start fields in the fall or spring from greenhouse grown plugs set 15-25 cm (6-10 in) apart within row. Fertilization and irrigation: Typically, no fertilizer is applied until May after new fall plantings when 27-44 kg of nitrogen/ha (25-40 lbs N/ac) is used. For established seed fields of California oatgrass in western Oregon, annual applications of nitrogen are made in late February or March at rates of 55-110 kg N/ha (50-100 lbs N/ac). Suggested rates may change as more information is learned. Other fertilizers containing potassium, phosphorus, sulfur, or micronutrients may be needed according to soil tests. In western Oregon, no irrigation is required for new stands as long as the planting of seedlings or seeding is done in fall as recommended. Spring plantings will require irrigation the first year only. However, on droughty soils or in the Central Valley of California and other summer dry, low precipitation areas, summer irrigation may be needed every year. Weed and pest control: Weeds are controlled in new and existing stands by tillage, mowing, hand hoeing, spot or shielded spray treatments between rows with glyphosate herbicide, and applications of broadleaf herbicides with general labels for grass seed production (Peachy et al. 2007). Mowing off taller weeds and their flower heads that overtop the California oatgrass the first year provides some control. A better option is to use a flail forage harvester that both mows off and removes weed seed stalks, including annual bluegrass (Poa annua). While a number of herbicides are labeled for control of this and other annual weedy grasses in established fields of perennial grasses grown for seed, only one of these products (dimethenamid-P) can be legally applied to native California oatgrass in Oregon (BASF Corporation 2007). Supplemental labeling expires December 31, 2009 unless renewed. Significant disease pests such as rust (Puccinia spp.), ergot (Claviceps purpurea), or smut (Ustilago spp.) and insect problems have not been reported. Always read and follow label directions completely when applying any herbicide or other pesticide. Harvesting: Harvesting California oatgrass is usually done by swathing (windrowing) followed by combining a week or two later after the seed and stalks have adequately dried. It is important to harvest the hidden seed (cleistogenes) in the stems since their numbers are often greater than the amount of seed produced in the exposed inflorescence. To extract both types of seed in a single operation, some growers (Kenagy 2007, Anderson 2008) use a combine equipped with an aggressive four row, spike toothed cylinder and concave set at narrow clearance. Other options include double harvesting using a combine with a rasp cylinder or stripping and vacuuming the seed with a flail-vac seed stripper. The stripper, mounted like a front end loader on a tractor, has a fast spinning brush which rips, pulls, or vacuums the seed from the seed heads and throws it into a hopper. Newer forms of seed strippers may work as well. However, stripping wastes hidden seed unless stems are harvested separately. This can be done later in summer when they readily break off near the base of the plant. The additional seed is then extracted with an aggressive stationary seed thresher or hammer mill that breaks apart the stems. Flowering and seed formation is commonly absent or meager the first year. This may be due to the plant’s slow development, vernalization requirement, or both. Yields increase in subsequent years and often will not peak until the third growing season. They can average 110-330 kg/ha (100-300 lbs/ac). Properly managed, fields can remain productive for a decade or more. Crop residues: Post harvest residue management usually involves simple mowing to remove decadent foliage and improve exposure of grass crowns and growing points to light and cool temperatures, or flail chopping to break up the stems and leaf matter into finer material. Excessive plant litter left on the soil surface can reduce the effectiveness of certain herbicides in grass seed production, so baling heavier residues, if present, may also be a good option. A flail forage harvester will both cut and remove the crop aftermath in one operation. Given the general tolerance of California oatgrass to fire, summer burning during plant dormancy may be an alternative but no information on its application or benefit for seed production of this species has been reported. Seed cleaning and conditioning: Threshed seed is usually first run through a scalping machine or screen to remove stems and other course materials. As described earlier, dehulling/scarification of some seed lots can improve germination if excessive seed damage can be avoided in the process. Options include using a huller/scarifier (brush machine) equipped with gentler brushes or run at slower speeds, or possibly an oat dehuller. Seed is often de-awned in the same process which may be a goal in itself to improve flow through certain seeding equipment and reduce storage volume. Final cleaning is done with an air-screen machine but care is needed to prevent the wasteful disposal of longer cleistogenes or smaller dehulled seed (groats or kernals). In Oregon, seed certification standards for California oatgrass require a minimum purity of 90% and allow a maximum of 0.15% other crops, 10% inert matter, 0.15% common weed seeds, and four restricted weed seeds/lb (Oregon Seed Certification Service 2008). Plant production: Containers or flats of California oatgrass can be sown in winter or early spring with untreated, dry seed presumably low in dormancy (Young 2001, Dyer 2001) or fresh seed sown immediately after harvest before dormancy develops (Amme 2008). Standard, well drained potting media amended with micronutrients and optional starter or slow release fertilizers works well. Others suggest a medium of 1:1 peat and vermiculite and a light application of nitrogen fertilizer weekly (Rose et al. 1998) or every six weeks with 10-10-5 NPK soluble fertilizer (Amme 1986). Sometimes the seed is dehulled/scarified then sown in plastic flats lined with newspaper and kept at temperatures from 15-25°C (59-75°F) (Keeley 2000). Several shapes and sizes of plug type containers are used for production of seedling transplants. The seed should be covered with 0.6 cm (0.25 in) or less of potting media or vermiculite and kept moist. Germination and sprouting commences in 10-21 days, but can continue for months (Amme 1986). Plants are maintained with irrigation under controlled greenhouse conditions at 18-25°C (65-75°F). Fertilization is typically discontinued during the summer months. Compared to fall and winter sown and potted seedlings (liners), those similarly handled in late spring and early summer had few or no flowering culms by fall (Amme 1986). Cutting the plants back once or twice helps prevent containers from drying out and encourages new growth of culms. If held over, a single clipping maintained good vigor for California oatgrass through the second year (Amme 1986). For seed lots where physiological dormancy is initially high, flats or containers are fall sown at the Corvallis PMC with untreated or partially dehulled seed and left outdoors over winter to naturally stratify. Alternatively, flats or trays of plugs are sown, well watered, placed in plastic bags, and moved into a walk-in cooler for cold moist stratification at 1-4°C (34-40°F) for 90-120 days. If the principal dormancy is seed-coat imposed, it is suggested that seed be carefully acid scarified or dehulled in advance and then sown soon after at any time of the year. Once in the warm greenhouse and seedlings emerge, it can take 9-12 weeks for plants to become well established in 7-10 cubic inch, cone shaped containers. Plants should be acclimated for several weeks or more in a lath house or shadehouse prior to spring outplanting or maintained there until fall. For seed with physiological dormancy, remaining “empty” containers may be held over until next spring as they often contain viable seed that will germinate after a second winter period. California oatgrass propagates readily by division. One method is to collect plants during the dormant season or maintain them in a lath house until dormant. Bring the clumps into a greenhouse in January, and divide them up into segments with a single root. Plantlets are then potted in plug or cone shaped containers, kept moist in the greenhouse at 18-21°C (65-70°F), and later moved back to a lath house (Dyer 2001, Rose et al. 1998). One gallon pots can be split into 3-5 plugs (Las Pilitas Nursery 2007). Cultivars, Improved, and Selected Materials (and area of origin) In 2000, the NRCS Plant Materials Center at Corvallis, Oregon, the US Fish and Wildlife Service, and the Oregon Agricultural Experiment Station released Baskett Slough Germplasm California oatgrass, a selected class pre-variety (Darris and Lambert, 2000). The origin of this “natural” germplasm is the Baskett Slough National
Wildlife
Refuge in Polk County, Oregon. It was not bred or hybridized and particular attention was given to include genetic diversity indicative of the population. Primarily for restoration and erosion control, its intended area of use is US EPA Level III Ecoregion 3 or the southern portion of USDA Major Land Resource Area (MLRA) 2 which includes the Willamette Valley of Oregon and a part of southwest Washington below 1500 ft. elevation. Seed is commercially available. Several other source identified populations of California oatgrass are periodically grown and sold as seed in Oregon and California. Native plant nurseries regularly produce plants of known origin in plugs and pots. Population genetics and seed transfer: Understanding patterns of genetic variation in California oatgrass across the landscape provides insight into adaptation and can help guide seed movement of populations within and among regions. In an analysis of data from a common garden study containing 66 populations (accessions) primarily from western Oregon and southwest Washington, plant vigor and seed abundance significantly correlated with winter precipitation, winter minimum temperature, and summer maximum temperature. However, for the subset of 33 Willamette Valley accessions, there were no significant correlations suggesting this region could be treated as a single seed zone for the species (Johnson undated). In an evaluation of isozyme (protein enzyme) systems in 22 populations of California oatgrass from Oregon and California, Knapp and Rice (1994) found higher levels of among-population variation than within. Such a pattern is more typical of self-pollinating species unlike open–pollinated conifers which tend to exhibit the opposite. This makes it more likely for California oatgrass to have genetically distinct populations resulting in the need for smaller seed transfer zones compared to tree species. In addition, variety californica and variety americana were found to have distinct genetic compositions. As a result, for purposes of restoration, they advise against mixing the two varieties and are in favor of matching each variety to the variety growing in the vicinity. However, most taxonomic authorities do not recognize the two varieties as separate entities. Knapp and Rice also noted seedlings from one population with narrower leaves came from a unique vernal pool location suggesting that restorationists should consider localized selection pressures when considering seed sources for a planting.
References
Alderman, S.C. 2001. Blind seed disease. Miscellaneous Publication No. 1567. United States Department of Agriculture, Agricultural Research Service. URL: http://arsserv0.tamu.edu/is/np/ blindseed/contents.htm Amme, D. 1986. Nursery production of western native perennial grasses. In: Proceedings of Conference XVI International Erosion
Control
Association 1985. San Francisco, CA. Amme, D. 2003. Creating a native California meadow. Grasslands 13(3):1, 9-11. Amme (2008). Personal communication. California Native Grasslands Association and East Bay Parks. Anderson, J. 2008. Personal communication. Hedgerow Farms. Winters, CA. Angelo, C. 2005. Restoration of Danthonia californica, Elymus glaucus, and Nassella pulchra at Elkhorn Slough National Estuarine Reserve. University of California Santa Cruz. Santa Cruz, California. Bainbridge, S. & C. D’Antonio. 2003. Prescribed fire for controlling exotics in the California grassland: factors influencing success. Workshop: Use of fire to control invasive plants. 7th International Conference on the Ecology and Management of Invasive Species. Ft. Lauderdale, Florida. Bartolome, J.W., J.S. Fehmi, R.D. Jackson, & B. Allen-Diaz. 2004. Response of a native perennial grass stand to disturbance in California’s Coast Range grassland. Restoration Ecology. 12(2): 279-289, BASF Corporation. 2007. Outlook herbicide for use in perennial grasses grown for seed. Supplemental label. BASF Corporation. Research Triangle Park, NC. Boyer, L. 2007a. Personal communication. Heritage Seedlings. Salem, OR. Boyer, L. 2007b. Native Willamette Valley prairie and oak habitat restoration site preparation and seeding information. Heritage Seedlings. Salem, OR. URL: http://www.heritageseedlings.com/PDF/ prairieandoakrestorationmethods.pdf Brady, K.U., A.R. Kruckeberg, and H.D. Bradshaw Jr. 2005. Evolutionary ecology of plant adaptation to serpentine soils. Annual Review of Ecology. Evolution and Systematics 36:243–66. Bruns, E. 2005. Maternal investment in and fitness of chasmogamous and cleistogamous progeny of the native perennial grass Danthonia californica. Undergraduate thesis. University of California Santa Cruz. Buisson, E., E. Corcket, T. Dutoit, S. Anderson, & K. Holl. 2004. Restoring old fields by seeding and transplanting keystone species in the grasslands of California central coast. ESA Annual Meeting, Portland, OR. Ecological Society of America. Campbell. B.H. 2004. Restoring rare native habitats in the Willamette Valley. Defenders of Wildlife, West Linn, Oregon and Washington D.C. Campbell, C.S., J.A. Quinn, G.P. Cheplick, & T. J. Bell. 1983. Cleistogamy in grasses. Annual Review of Ecology and Systematics 14: 411-441. Clark, D.L. & M.V. Wilson. 2005. Restoration of native upland prairies for Fender’s blue butterfly (Icaricia icarioides fenderi). Report for Oregon Fish and Wildlife Service and U.S. Fish and Wildlife Service, Portland, Oregon. Chappell, C.B. 2006. Upland plant associations of the Puget Trough ecoregion, Washington. Natural Heritage Rep. 2006-01. Washington Department of Natural Resources, Natural Heritage Program, Olympia , WA URL: http://www.dnr.wa.gov/nhp/refdesk/communities/pdf/intro.pdf Chirco, E & T. Turner. 1986 (updated 2007). Species without AOSA testing procedures. The Newsletter of the Association of Official Seed Analysts. Vol. 60, 2:2-66 Collins, M. 2006. Designation of critical habitat for the Fender's blue butterfly (Icaricia icarioides fenderi), Lupinus sulphureus ssp. kincaidii (Kincaid's lupine), and Erigeron decumbens var. decumbens (Willamette daisy); Final Rule. U.S. Fish and Wildlife Service. Federal Register: October 31, 2006.71(210): 63861-63977. Cooper, D.W. 1960. Fort Baker ranges return to champagne grasses. Journal of Range Management 13:203-205. Crampton, B. 1974. Grasses in California. California Natural History Guides 33. University of California Press. Berkeley, California. Daniels, S. 2007. Native grasses suitable for lawn substitutes. Wild Lawns Inc. URL: http://ww2.lafayette.edu/~danielss/grasses.html Darbyshire. S.J. 2003. Danthonia. In: Flora of North America Editorial Committee, eds. Flora of North America North of Mexico. 12+ vols. New York and Oxford. Vol. 25. 301-306. Darris, D.C. 2003. Considerations for establishing native grasses from seed for restoration, revegetation and erosion control in western Washington and western Oregon. Plant Materials Technical Note No. 35. USDA Natural Resources Conservation Service. Portland, Oregon. Darris D. and S. Lambert. 2000. Baskett Slough Selection of California oatgrass. Fact Sheet. USDA Natural Resources Conservation Service. Plant Materials Center, Corvallis, OR. Dobrenz, A.K., & A.A. Beetle. 1966. Cleistogenes in Danthonia. Journal of Range Management. 19:292-296. Drewitz, J. & J. Anderson. 2003. Using herbicides to control weeds in native grass plantings. Section 15 IN: Using native grasses and graminoids in restoration and revegetation. Workshop proceedings. California Native Grasslands Association. Davis CA. Dyer, D.A. 2001. Propagation protocol for production of container Danthonia californica; USDA NRCS Lockeford Plant Materials Center, Lockeford, CA. In: Native Plant Network. URL: http://www.nativeplantnetwork.org (accessed 10 December 2007). Moscow (ID): University of Idaho, College of Natural Resources, Forest Research Nursery. Edminster, C. 2003. California oatgrass Danthonia californica. Pacific Northwest Natives. Albany, Oregon. URL: http://www.pacificnwnatives.com/California_Oatgrass.pdf Edwards, S.W. 1992. Observations on the prehistory and ecology of grazing in California. Fremontia 20:3-11. Emery, D.E. 1988. Seed propagation of native California plants. Santa Barbara Botanic Garden. Santa Barbara, CA. Fire Safe Council. 2007. Fire safe vegetation. El Dorado County, California. URL: http://www.edcfiresafe.org/fire_safe_vegetation.htm#nativeVines (accessed 12/18/2007). Fischer, G.W. 1944. The blind-seed disease of ryegrass (Lolium sp.) in Oregon. Phytopathology 34:934-935. Franklin J.F. and C.T. Dyrness. 1973. Natural Vegetation of Oregon and Washington.
General
Technical Report PNW-8. USDA Forest Service. Portland, Oregon. Guerrant, E.O. Jr. and A. Raven. 1995. Seed germination and storability studies of 69 plant taxa
Conservation
Biology 17(6): 1694-1702. Heady, H.F., D.W. Cooper, J.M. Rible, & J.F. Hooper. 1963. Comparative forage values of California oatgrass and soft chess. Journal of
Range
Management 16:51-54. Heritage Seedlings. 2007. Native Willamette Valley seed. Salem, Oregon. URL: http://www.heritageseedlings.com/PDF/SeedPriceList.pdf Hickman, J.C. (editor) 1993. The Jepson Manual: Higher Plants of California. University of California, Berkeley and Los Angeles, California. Hitchcock, C.L., A. Cronquist, M. Ownbey, & J.W. Thompson. 1969. Vascular plants of the Pacific Northwest. University of Washington Press, Seattle and London. Jebb, T. 1995. Personal communication. USDA Bureau of Land Management. Sprague Seed Orchard, Merlin, OR. Cited in: Rose, R., C.E.C. Chachulski & D.L. Haase. 1998. Propagation of Pacific Northwest Native Plants. Oregon State University Press. Corvallis, OR. Johnson, R. (undated) Summary of common garden studies: what do we know about genetic variation in the Willamette Valley? Pacific Northwest Research Station. USDA Forest Service. URL: http://www.nativeseednetwork.org/site_files/SummaryOfCommonGardenStudies_-_RandyJohnson.pdf Kanegy, P. 2007. Personal communication. Kanegy Seed Farm, Albany, OR. Keeley, M.A. 2000. A study in urban revegetation: germination and establishment of South Puget Sound prairie plants on a capped landfill. MS Thesis. University of Washington. Seattle, WA. Kephart, P. & D. Amme. 1992. Native perennial grass establishment and management. Grasslands. California Native Grass Association. Feb. 1992. Klinkenberg. B. (editor) 2007. E-Flora BC: electronic atlas of the plants of British Columbia [www.eflorea.bc.ca]. Lab for Advanced Spatial Analysis, Department of Geography, University of British Columbia, Vancouver (accessed 12/10/2007). Knapp, E. & K. Rice. 1994. Isozyme variation within and among populations of Danthonia californica: final report. Department of Agronomy and Range Science, University of California Davis. Davis, California. Kozloff, E.N. 2005. Plants of Western Oregon, Washington, and British Columbia. Timber Press. Portland, Oregon. Las Pilitas Nursery. 2007. Danthonia californica. URL: http://laspilitas.com/plants/459.htm (accessed 12/5/2007). Laude, H.M. 1949. Delayed germination of California oatgrass, Danthonia californica. Agron. J. 41:404-408. Maggenti, A.R., W.H. Hart & G.A. Paxman. 1974. A new genus and species of gall forming nematode from Danthonia californica, with a discussion of life history. Nematologica 19 (1973): 491-497. Maslovat, C.Y. 2001. Germination ecology of native grass species, Danthonia californica and Elymus glaucus, in Garry oak ecosystems and the implications for restoration. Master of Science Thesis. University of Victoria, Victoria, British Columbia, Canada. Maslovat, C.Y. 2002. Historical jigsaw puzzles: piecing together the understory of Garry oak (Quercus garryana) ecosystems and the implications for restoration. In: General Technical Report PSW-GTR-184. USDA Forest Service. McClaran, M.P. 1981. Propagating native perennial grasses. Fremontia. 9(1): 21-23. Menke, J.W. 1992. Grazing and fire management for native perennial grass restoration in California grasslands. Fremontia. 20(2):22-25. Mohlenbrock, R.H. 1992. Western wetland flora: field office guide to plant species. USDA Natural Resources Conservation Service. West Region, Sacramento, California. Mousseaux, M. 2004. French Flat. Kalmiopsis 11:46-53. NatureServe. 2008. NatureServe Explorer: An online encyclopedia of life [web application]. Version 7.0. NatureServe, Arlington, Virginia. URL: http://www.natureserve.org/explorer. (Accessed: April 7, 2008). Northwest Habitat Institute. 2008. Habitat descriptions. Interactive Biodiversity Information System. Corvallis, Oregon. URL: http://www.nwhi.org/index/ibis Oregon Department of Fish and Wildlife. 2000. Landowner’s guide to creating grassland habitat for the Western Meadowlark and Oregon’s other grassland birds. Salem, OR. Oregon Seed Certification Service. 2008. Pre-variety germplasm seed standards. Oregon State University. Corvallis, Oregon. URL: http://www.oscs.orst.edu/standards/pvg-seed.standards.doc Peachey, E., et al. 2007. (compilers) Pacific Northwest weed management handbook. Extension Services of Oregon State University, Washington State University, and University of Idaho. Robinson, G.S., P.R. Ackery, I.J. Kitching, G.W. Beccaloni & L.M. Hernández. 2007. HOSTS-a database of the world's Lepidopteran hostplants. The Natural History Museum, London, England. URL: http://www.nhm.ac.uk/research-curation/projects/hostplants/#9 Rodgers, D. 1981. Notes on planting and maintenance of bunchgrasses. Fremontia. 9(1):24-28. Rose, R., C.E.C. Chachulski, & D.L. Haase. 1998. Propagation of Pacific Northwest natives. Oregon State University Press. Corvallis, OR. Santa Cruz Public Libraries (compiler). 2003. Ohlone tiger beetle. Endangered species in Santa Cruz County. URL: http://scplweb.santacruzpl.org/ref/endang/endang.shtml Simpson, G.M. 1990. Seed dormancy in grasses. Cambridge University Press. Cambridge and other cities. Stewart, H. & R.J. Hebda. 2000. Grasses of the Columbia Basin of British Columbia. Working Paper 45. Royal British Columbia Museum, Natural History Section. Victoria, BC, Canada. Stromberg, M.R. & P.H. Kephart. 2003. Landowners guide to native grass enhancement and restoration. Hastings Natural History Reservation, University of California, Carmel Valley, California. URL: http://www.hastingsreserve.org/NativeGrass/GrassManageIntro.html Stromberg, M. R., P. H. Kephart, & M. Sicular-Mertens. 2002. Restoration of native grasses in California old fields II: cheap tills. Unpublished Manuscript. Stromberg, M.R., P.H. Kephart, & V. Yadon. 2001. Composition, invasibility, and diversity in coastal California grasslands. Madrono. 48(4): 236-252. Suttle, K.B. & M.A. Thornsen. 2007. Climate change and grassland restoration in California: lessons from six years of rainfall manipulation in a north coast grassland. Madrono. 54 (3):225-233. Trask, M. 1996. Germination enhancement trials pilot project. Grasslands. VI(1):1-3. Trask, M.M. & D.A. Pyke. 1998. Variability in seed dormancy of three Pacific Northwestern grasses. Seed Science Technology. 26:179-191. Tu, M., C. Hurd, & J.M. Randall. 2001. Weed control methods handbook: tools and techniques for natural areas. The Nature Conservancy. URL: http://tncweeds.ucdavis.edu/handbook.html USDA Forest Service. 1988 (reprint). Range Plant Handbook. Dover Publications, Inc. New York. USDA, NRCS. 2007. The PLANTS Database (http://plants.usda.gov, 10 December 2007). National Plant Data Center, Baton Rouge, LA 70874-4490 USA. Weatherwax, P. 1928. Cleistogamy in two species of Danthonia. Botanical Gazette. 86: 104-109. Wilson, M.V. & D.L. Clark. 2001. Controlling invasive Arrhenatherum elatius and promoting native prairie grasses through mowing. Applied Vegetation Science 4.1:129-138. Wrysinski, J. 2004. Know your natives. A pictorial guide to California native plants. Yolo County Resource Conservation District. Woodlands, California. Young, B. 2001. Propagation protocol for production of container Danthonia californica Boland plants; USDI NPS – Golden Gate National Park, San Francisco, CA. In: Native Plant Network. URL: http://nativeplantnetwork.org (accessed 10 December 2007). Moscow (ID): University of Idaho, College of Natural Resources, Forest Research Nursery.
Plant Traits
Growth Requirements
| Temperature, Minimum (°F) | 17 |
|---|---|
| Adapted to Coarse Textured Soils | No |
| Adapted to Fine Textured Soils | Yes |
| Adapted to Medium Textured Soils | Yes |
| Anaerobic Tolerance | None |
| CaCO3 Tolerance | Medium |
| Cold Stratification Required | No |
| Drought Tolerance | Medium |
| Fertility Requirement | Low |
| Fire Tolerance | High |
| Frost Free Days, Minimum | 90 |
| Hedge Tolerance | None |
| Moisture Use | Medium |
| pH, Maximum | 7.0 |
| pH, Minimum | 6.0 |
| Precipitation, Maximum | 40 |
| Precipitation, Minimum | 6 |
| Root Depth, Minimum (inches) | 16 |
| Salinity Tolerance | Low |
| Shade Tolerance | Intolerant |
Morphology/Physiology
| Bloat | None |
|---|---|
| Toxicity | None |
| Resprout Ability | No |
| Shape and Orientation | Erect |
| Active Growth Period | Fall, Winter and Spring |
| Coppice Potential | No |
| Fall Conspicuous | No |
| Fire Resistant | Yes |
| Flower Conspicuous | No |
| Foliage Color | Green |
| Foliage Porosity Summer | Porous |
| Foliage Porosity Winter | Porous |
| Foliage Texture | Fine |
| Fruit/Seed Conspicuous | No |
| Growth Form | Multiple Stem |
| Growth Rate | Moderate |
| Height, Mature (feet) | 2.0 |
| Known Allelopath | No |
| Leaf Retention | No |
| Lifespan | Short |
| Low Growing Grass | Yes |
| Nitrogen Fixation | None |
| Fruit/Seed Color | Brown |
Reproduction
| Propagated by Seed | Yes |
|---|---|
| Propagated by Sod | No |
| Propagated by Sprigs | No |
| Propagated by Bare Root | No |
| Propagated by Tubers | No |
| Seed per Pound | 40000 |
| Seed Spread Rate | Moderate |
| Seedling Vigor | High |
| Small Grain | No |
| Vegetative Spread Rate | Moderate |
| Propagated by Corm | No |
| Propagated by Container | No |
| Propagated by Bulb | No |
| Fruit/Seed Persistence | No |
| Fruit/Seed Period End | Spring |
| Fruit/Seed Period Begin | Spring |
| Fruit/Seed Abundance | Medium |
| Commercial Availability | Routinely Available |
| Bloom Period | Early Spring |
| Propagated by Cuttings | No |
Suitability/Use
| Veneer Product | No |
|---|---|
| Pulpwood Product | No |
| Protein Potential | Medium |
| Post Product | No |
| Palatable Human | No |
| Palatable Graze Animal | High |
| Palatable Browse Animal | Low |
| Nursery Stock Product | No |
| Naval Store Product | No |
| Lumber Product | No |
| Fodder Product | Yes |
| Christmas Tree Product | No |
| Berry/Nut/Seed Product | No |