Chinese Tallow
Scientific Name: Triadica sebifera (L.) Small

| General Information | |
|---|---|
| Usda Symbol | TRSE6 |
| Group | Dicot |
| Life Cycle | Perennial |
| Growth Habits | Tree |
| Native Locations | TRSE6 |
Plant Guide
Alternate Names
This plant appears in most floras as Sapium sebiferum. Common names include popcorn tree, chicken tree, and Florida aspen.
Uses
Ingestion of plant material causes gastrointestinal upset with nausea and vomiting. Contact with the plants can cause dermatitis (Westbrooks & Preacher 1986). The milky sap in both the leaves and the berries is poisonous to animals (Redlus 1997). Sheep and goats have been known to eat the leaves of Chinese tallow, but the plant is toxic to cattle (Jubinsky & Anderson 1996; Russell et al. 1969). Chinese tallow is very invasive. The Chinese tallow tree was introduced into the United States from China in 1776 by Ben Franklin (Randall & Marinelli 1996). The common name comes from the waxy tallow derived from the white covering of the seed that has been used historically to make soap and candles. It has been cultivated in China for at least 14 centuries as a seed crop and as an ornamental. In the early 1900s, the Foreign Plant Introduction Division of the U.S. Department of Agriculture promoted tallow tree planting in Gulf Coast states to establish a local soap industry (Flack & Furlow 1996). Since that time, the species has been planted in the U.S. mainly for its unique ornamental qualities including colorful, autumnal foliage. © L. Urbatsch The plants have tremendous reproductive potential. They may reach reproductive age in as little as three years and remain productive for 100 years (Duke 1983). In greenhouse experiments, some trees grown from seed flowered in their first year of growth (Grace 1997). A mature tree may annually produce an average of 100,000 seeds that are spread mainly by birds and water (Duke 1983; Jubinsky & Anderson 1996). Stumps have the ability to resprout and roots readily develop shoots. The species causes large-scale ecosystem modification by replacing native vegetation thereby reducing native species diversity that, in turn, has a negative effect on wildlife (Bruce et al. 1995; Randall & Marinelli 1996). The ability of this species to drastically modify natural landscapes has earned it a spot on The Nature Conservancy’s list of "America’s Least Wanted -The Dirty Dozen" (Flack and Furlow 1996). The species can readily become the dominant plant in disturbed vacant lots and abandoned agricultural land. It has the ability to invade natural, wet prairies and bottomland forests. Once established, it is virtually impossible to eliminate by known methods, and efforts to achieve effective control are being explored (Jubinsky & Anderson 1996). Jubinsky & Anderson (1996) demonstrate the invasive nature of Chinese tallow in Florida. Their work has shown Chinese tallow has become naturalized in over half (57%) of Florida’s counties. Vegetation analysis demonstrates its potential dominance in Florida’s wetlands. Quantification of trees at their study site revealed that Chinese tallow had the greatest density of all woody plants despite being present for only 20 years. In relative frequency, it was second to sweetgum (Liquidambar spp.). Compared to other woody species, Chinese tallow was found to reproduce prolifically. Beneath tallow trees of reproductive age, 14.6 seedlings were present per square meter. Percent cover of seedlings of Chinese tallow was significantly greater than all other woody species combined. Relative frequency of tallow seedlings was also greater than that of all species except for sweetgum. Native species negatively impacted at the site-included sweetgum, various species of oak (Quercus spp.), black willow (Salix nigra), swamp dogwood (Cornus foemina), and elderberry (Sambucus canadensis). Chinese tallow is the most successful exotic invader of the chenier woodlands in southwestern Louisiana. It has had a major impact on plant community structure and species composition by becoming the most abundant woody species. It has the potential to invade surrounding marshes, changing them from herbaceous to woody plant communities (Neyland & Meyer, 1997). In Texas, the introduction of tallow trees has produced large-scale conversion of much of the upper coastal prairie to woodland (Bruce et al. 1995). Presettlement vegetation was dominated by little bluestem (Schizachyrium scoparium) and other typical prairie species. The new, anthropogenic woodlands are essentially monospecific stands of tallow trees with some native tree species. Besides altered species composition, Chinese tallow woodlands have altered ecosystem processes. Their primary productivity is higher, and ion concentrations in the soil have been changed (Bruce et al. 1995). Tallow tree woodland is expected to either persist as a novel, stable, exotic vegetation type or to gradually give way to a novel type dominated by native species (Bruce et al. 1995). Regardless of which scenario takes place, this species has supplanted naturally occurring prairie where it has invaded. Associated animal species are dramatically affected by such habitat modifications. Attwater’s prairie chicken (Tympanuchus cupido attwateri), once abundant in the coastal prairie of Texas and Louisiana, is near extinction due to loss of habitat. Other bird species dependent on coastal prairie habitat are dickcissels, mottled ducks, and a large number of neotropical migratory songbirds including five critically imperiled species. Data indicate that a dramatic decline has occurred over the past 25 years in grassland bird species (Knopf 1994). To determine its potential to invade and persist in hardwood forests, the shade tolerance of Chinese tallow was compared to American sycamore (Platanus occidentalis) and cherrybark oak (Quercus pagoda) in a series of experiments (Jones & McLeod 1989). Tallow tree growth was shown to exceed sycamore and oak in shade, and tallow tree and sycamore growth exceeded oak in full sun. Such findings are consistent with observed patterns of tallow tree growth in North America. With its ability to grow rapidly in sunlight or under closed canopies, the species is likely to invade and persist in many of the floodplain forests of the coastal Southeast. © L. Urbatsch Cross-section of seed showing tallow portion. The precise mechanism of spread remains somewhat open to question. Chinese tallow trees are reported to have allelopathic capabilities in that the leaves contain substances that alter the soil chemistry and negatively impact native vegetation (Flack & Furlow 1996). However, Conway et al. (1997) investigated the effects of tallow leaf-litter water extracts on the germination of bald cypress (Taxodium distichum) and black willow (Salix nigra), and found no evidence for allelo-chemical effects. They concluded that suppression of associated species would be expected due to shading and other methods of resource reduction, and if present, allelopathy contributes less significantly to tallow’s spread and dominance in habitats that were once more natural. Wildlife: The flowers of Chinese tallow are favored by honeybees, which produce a desirable, light-colored honey from it. Birds, including domestic fowl readily eat its fruits. It has been considered a desirable plant for bird-food (Duke 1983). Oil: Chinese tallow tree seeds are a source of vegetable tallow, a drying oil, and protein food. The tree is also planted for ornamental purposes. The outer covering of the seeds contains solid fat known as Chinese vegetable tallow and the kernels produce a drying oil called Stillingia oil. Candles, soap, cloth dressing, and fuel are made from the tallow. The oil is used in machine oils, as a crude lamp oil, and in making varnishes and paints, because of its quick-drying properties. Also, the oil reportedly is used in Chinese medicine, but overdoses probably would cause violent sickness and perhaps death (Duke 1983). After the seeds have been processed, the residual cakes are used as manure. Biomass: More recently, the tree has been regarded as a promising biomass candidate in the Gulf coast region of the United States, because of its ability to re-sprout, its rapid growth rate, and its drought and salt tolerance (Scheld & Cowels 1981). Field trials demonstrate that the species can produce six times as much woody biomass as Populus selections grown in Wisconsin (Scheld & Cowles 1981). Chinese tallow can be grown over large areas by conventional agricultural methods and can provide woody biomass for direct burning or conversion to charcoal, ethanol, and methanol (Scheld & Cowles 1981). Oil from the seeds can potentially substitute for petroleum. Scheld et al. (1980) report yields of 4,000 to 10,000 kg/ha, and cite estimates of 25 barrels of oil per year as a sustained energy yield. In addition to its biomass and energy value, the tree is extensively propagated for ornamental purposes. From 200,000-300,000 trees are grown for ornamental purposes in Houston, Texas alone (Duke 1983). Construction: Tallow wood is white and close-grained, suitable for carving and for making blocks in Chinese printing. The wood is also used for furniture making and incense. A black dye can be made by boiling leaves in alum water (Duke 1983).
Status
Please consult the PLANTS Web site and your State Department of Natural Resources for this plant’s current status, such as, state noxious status, and wetland indicator values.
Description
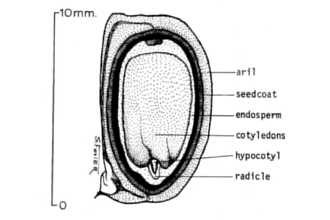
General: Spurge Family (Euphorbiaceae). Chinese tallow is a fast-growing, medium-sized tree that may reach heights of 50 feet (Godfrey 1988). This noxious plant causes large-scale ecosystem modification throughout the southeastern U.S. by replacing native vegetation. It quickly becomes the dominant plant in disturbed vacant lots, abandoned agricultural land, natural wet prairies, and bottomland forests. Once established, Chinese tallow is virtually impossible to eliminate. Tallow leaves are alternate (one per node), with broad rhombic to ovate blades, 1.5-3.5 inches long, 1.5-4 inches wide, the leaf blade bases are wedge-shaped and the apex supports a gradually tapering appendage, margins are entire (without teeth). The upper leaf surface is dark green and the lower is somewhat paler. The veins are yellow and conspicuous on both surfaces. In autumn, the leaves turn orange to scarlet (sometimes yellow) and fall as the cold season approaches. The leaf stalks (petioles) are 1-4 inches long, with 2 swollen glands on the upper side immediately below the leaf blade. At the base of the petiole is a pair of stipule-like appendages about one-eighth inch long. © L. Urbatsch Glands at base of leaf. Chinese tallow trees are monoecious (i.e., an individual tree has separate pollen and seed bearing flowers). The pollen producing (staminate) flowers occur in greenish-yellow, tassel-like spikes up to 8 inches long that terminate the branchlets, and each flower cluster along the spike has a basal pair of nectar glands. A few seed producing (pistillate) flowers (each having a three lobed ovary and three style branches) are located on short branches at the base of the staminate spike. The pistillate flowers mature into three-lobed, three-valved capsules about 1/2 - 3/4 inch long and about 3/4 inch wide. As the capsules mature, their color changes from green to nearly black. When mature, the capsule walls fall away typically exposing three globose seeds that have a white, tallow-containing covering. Seeds usually persist on the plants for a period of weeks. Flowers typically mature April-June and fruit ripens in September-October. Distribution: Chinese tallow is native to many provinces of central China, especially north of the Yangtze Valley, and Japan (Duke 1983). It has been cultivated for 14 centuries as a seed crop (Duke, 1983; Jubinsky & Anderson 1996) and is presently grown in mainland China, Hainan Island, Hong Kong, Japan, Taiwan, and Korea. Chinese tallow has been introduced to many parts of the world including Sri Lanka, Indochina, Bengal, India, Sudan, Martinique, southern United States, southern France, and Algeria (Duke 1983; Jubinsky & Anderson 1996). Adaptation: Chinese tallow is naturalized in the United States from Cameron and Hidalgo counties in southernmost Texas northward to southern Oklahoma and northwestern Arkansas eastward to North Carolina and Florida. The species is very adaptable and can thrive in various environments. It is generally found in low, swampy places, and along the margins of bodies of fresh water; moreover, it can invade dry uplands as well, and it will tolerate some salinity. Tallow trees grow best in full sunlight but can tolerate shade. The spread of Chinese tallow appears to be limited by frigid and/or arid conditions (Jubinsky & Anderson 1966). Though widely planted as a street and ornamental tree in California, it has yet to become a pest there, presumably because of insufficient rainfall. For current U.S. distribution, please consult the Plant Profile page for this species on the PLANTS Web site. Vectors: The major dispersal methods of Chinese tallow are birds and water (Duke 1983; Jones et al. 1989). Jubinsky (1993) observed seeds being consumed and dispersed by pileated woodpeckers (Dryocopus pileatus) and boat-tailed grackles (Quicalus major) in Florida. Cardinals (Cardinalis cardinalis), and probably many other species of birds, also consume and presumably disperse the seeds (Skinner 1998). Seeds were also observed along riverbanks in central and north Florida after heavy rains, once higher waters receded (Jubinsky & Anderson 1996).
Control
Please contact your local agricultural extension specialist or county weed specialist to learn what works best in your area and how to use it safely. Always read label and safety instructions for each control method. Trade names and control measures appear in this document only to provide specific information. USDA, NRCS does not guarantee or warranty the products and control methods named, and other products may be equally effective.
Manual and Mechanical Control
Mechanical Control
Mechanical Control
Hand removal of trees is limited to trees usually less than three feet tall or to small areas. Sawing down large trees will help to remove seed sources. However, fruit should be removed from fallen trees in order to reduce the number of seeds present at the site. In order to prevent resprouting, stumps will require herbicide treatment. Heavy equipment can be effectively used to control tallow trees on canal banks and in areas where soil disturbance and non-selective species removal are not important considerations. Stumps remaining following such treatment will require herbicide application to prevent re-growth from cut surfaces. Environmental/Cultural Control In certain situations, altering the environment, such as raising or lowering the water levels, can cause sufficient stress to kill the plants (Jubinsky & Anderson 1996). Fire is another possible control means in appropriate habitats. Fire can have various effects on tallow trees: (1) complete kill, preliminary data suggest that this is unlikely; (2) top kill where the aerial portions of the trees are killed but re-sprouting occurs from the base, and (3) incomplete top-kill where the crown can re-sprout after fire. Preliminary data on the use of fire to control tallow trees in wet, coastal prairie appear to be limited. Only 17% of tree dominated plots burned compared to drier sites where 71% of the trees were exposed to fire. In areas with sufficient fuel, such as in prairies with good grass cover, summer burns killed or top killed trees as tall as three meters. However, control of trees growing in wet sites where fuels were poor was much less effective. Additional experimental work is being done on the effect of fire on tallow trees (Grace 1997).
Chemical Control
When considering chemical control, local laws affecting herbicide use must be observed, and care must be taken to avoid damage to non-target species. The various methods for applying herbicide to woody vegetation include basal bark, foliar, frill, or girdle treatments (Jubinsky & Anderson 1996). Basal bark treatment reportedly is the most effective for tallow trees (Jubinsky & Anderson 1996; Randall & Marinelli 1996). Several organizations including the Florida Department of Environmental Protection, the Florida Exotic Pest Plant Council, Florida Native Plant Society, Florida Nature Conservancy, and the Texas Nature Conservancy, have adopted this method of treatment (Jubinsky & Anderson 1996). Effective treatment consists of spraying a band at least six inches wide around the lowest 12 to 24 inches of the trunks with a triclopyr herbicide (Garlon ® 4; DowElanco trademark) at a concentration of about 15%. Concentrations up to 20% might be required for larger trees. A spray adjuvant formulated to mix with oil-soluble herbicides, such as JLB OIL PLUS or vegetable oil products are effective diluents (Kline & Duquesnel 1996; (13); Jubinsky & Anderson 1996). Pathfinder®II and Chopper® herbicides (DowElanco trademarks) are ready-to-apply basal-bark products. Such treatment can be done at any time of the year. Complete control of foliage, stems, and roots is possible with this method (Kline & Duquesnel 1996). For cut surface treatment, excellent results are reported with a 50% solution of Garlon 3A or with a 10% solution of imazapyr herbicide (Arsenal®, American Cyanamid trademark). The latter is a soil-active herbicide that requires careful use when applied near desirable plants or trees (Kline & Duquesnel 1996). A colorant is often added to the herbicide mix to aid in treatment monitoring, especially if done on a contractual basis. Examples of suitable dyes for this purpose include Blazon Blue Spray Pattern Indicator and Bullseye Spray Pattern Indicator (Kline & Duquesnel 1996).
Biological Control
Chinese tallow trees are remarkably free of insect pests and serious pathogenic organisms. This precludes the use of biological controls or integrated pest management programs. A few organisms known to associate with tallow trees include the bagworm, Eumeta, which feeds on the leaves. The root-knot nematode, Meloidogyne javanica, has been reported. Fungi known to attack this tree include Cercospora stillingiae, Clitocybe tabescens, Dendrophthoe falcata, Phyllactina corylea, Phyllosticta stillingiae, and Phymatotrichum omnivorum (Duke 1983). Illustrations and Photographs Godfrey, R. K. 1988. Trees, shrubs, and woody vines of northern Florida and adjacent Georgia, and Alabama. The University of Georgia Press, Athens, Georgia. 734 pp. (excellent line drawing showing leaves, flower, fruit, and seeds in detail, p. 284). Hunter, C. G. 1989. Trees, shrubs, and vines of Arkansas. The Ozark Society Foundation, Little Rock, Arkansas. p. 207. (photograph of a fruiting branch of tallow tree. p. 116). Radford, A. E., H. E. Ahles, & C. R. Bell 1968. Manual of the vascular flora of the Carolinas. University of North Carolina Press, Chapel Hill, North Carolina. (small line drawing, p. 666). Randall, J. M. & J. Marinelli 1996. Invasive plants, weeds of the global garden. Brooklyn Botanic Garden, Handbook #149, Brooklyn, New York. 99 p. (photograph of the seeds and the tree in flower, p. 41). Schopmeyer, C. S. 1974. Seeds of woody plants in the United States. USDA, Forest Service, Agricultural Handbook No. 450. 883 p. (photograph and illustration of seeds of Triadica sebifera, p. 760). Vines, R. A. 1960. Trees, shrubs, and woody vines of the southwest. University of Texas Press, Austin, Texas. 1104 p. (illustration of a leafy shoot with fruit pg. 622). Westbrooks, R. G. & J. W. Preacher 1986. Poisonous plants of eastern North America. University of South Carolina Press, Columbia, South Carolina. 172 p. (photograph showing the seeds and the tree in flower, p. 106).
References
Bruce, K, A,, G, N, Cameron, & P, A, Harcombe 1995, Initiation of a new woodland type on the Texas coastal prairie by the Chinese tallow tree (Sapium sebiferum (L,) Roxb,), Bulletin of the Torrey Botanical Club 122:215-225, Cameron, G, Use soil moisture sensors to measure the soil moisture of Chinese Tallow., N, & T, W, LaPoint 1978, Effects of tannins on the decomposition of Chinese tallow leaves by terrestrial and aquatic invertebrates, Oecologia 32:349-366, Cameron, G, N, & S, R, Spencer 1989, Rapid leaf decay and nutrient release in a Chinese tallow forest, Oecologia 80:222-228, Conner, W, H, 1994, The effect of salinity and waterlogging on growth and survival of baldcypress and Chinese tallow seedlings, Journal of Coastal Research 10:1045-1049, Conway, W, C,, L, M, Smith, & J, F, Bergan 1997, The allelopathic potential of an exotic invasive tree: Chinese tallow, Bulletin of the Ecological Society of America 78:72, Correll, D, S, & M, C, Johnston 1970, Manual of the vascular plants of Texas, The Texas Research Foundation, Renner, Texas, p, 1881, Cowles, J, R, & H, W, Scheld 1987, Cultural and management practices for the Chinese tallow tree as a biomass fuel source, Oak Ridge National Laboratory, ORNL/Sub/81-09059/1, 49 p, Diamond, D, D, & F, E, Smeins 1984, Remnant grassland vegetation and ecological affinities of the upper coastal prairie of Texas, Southwestern Naturalist 29:321-334, Duke J, A, 1983, Handbook of energy crops, unpublished, (2 October 1996), Flack, S, & E, Furlow 1996, America's least wanted "purple plague," "green cancer" and 10 other ruthless environmental thugs, Nature Conservancy Magazine, Vol, 46, No, 6 November/December, Grace, J,B, 1977, Personal communications, Research Ecologist, USDI, GS, National Wetlands Research Center, Lafayette, Louisiana, Godfrey, R, K, 1988, Trees, shrubs, and woody vines of northern Florida and adjacent Georgia and Alabama, University of Georgia Press, Athens, Georgia, p, 734, Harcombe, P, A,, G, N, Cameron, & E, G, Glumac 1993, Above-ground net primary productivity in adjacent grassland and woodland on the coastal prairie of Texas, USA, Journal of Vegetation Science 4:521-530, Hunt, K, W, 1947, Charleston woody flora, Amer, Midl, Naturalists 37:670-786, Jamieson, G, S,, & R, S, McKinney 1938, Stillingia oil, Oil and Soap 15:295-296, Jones, R, H, & K, W, McLeod 1989, Shade tolerance in seedlings of Chinese tallow tree, American sycamore, and cherry bark oak, Bull Torrey Bot, Club 116:371-377, Jubinsky, G, 1993, A review of the literature: Sapium sebiferum Roxb, Report No, TSS 93-03, Florida Department of Natural Resouces, Bur, Aq, Plant Management, Tallahassee, Florida, Jubinsky, G, & L, C, Anderson 1996, The invasive potential of Chinese tallow-tree (Sapium sebiferum Roxb,) in the southeast, Castanea 61:226-231, Kline, W, N, & J, G, Duquesnel 1996,
Management
of invasive exotic plants with herbicides in Florida. Down to Earth. Vol 51. No. 2. Also available at: http://fleppc.org/trtguide.htm Knopf, F. L. 1996. Prairie legacies - birds. IN: F. B. Sampson and F. L. Knopf (eds.). Prairie Conservation. Island Press, Washington, DC. Lin, W. C., A. C. Chen, C. J. Tseng & S. G. Hwang 1958. An investigation and study of Chinese tallow tree in Taiwan (Sapium sebiferum Roxb.). Bulletin of the Taiwan Forestry Research Institute 57:1-37. Neyland, R. & H. A. Meyer 1997. Species diversity of Louisiana chenier woody vegetation remnants. Journal of the Torrey Botanical Society 124:254-261. Princen, L. H. 1977. Potential wealth in new crops: Research and development. Pp. 1-15. IN: Seigler, D.S. (ed.), Crop resources Academic Press, Inc., New York, New York. Randall, J. M. & J. Marinelli 1996. Invasive plants, weeds of the global garden. Brooklyn Botanic Garden, Handbook #149, Brooklyn, New York. 99 p. Redlus, H. 1997. National animal poison control center information. (9 June 1997). Russell, L. H., W. L. Schwartz, & J. W. Dollahite 1969. Toxicity of Chinese tallow tree (Sapium sebiferum) for ruminants. American Journal of Veterinary Research 30:1233-1238. Scheld, H. W. & J. R. Cowles 1981. Woody biomass potential of the Chinese tallow tree Sapium sebiferum. Econ. Bot. 35:391-397. Scheld, H. W., N. B. Bell, G. N. Cameron, J. R. Cowles, C. R. Engler, A. D. Kridorian, & E. B. Schultz Jr. 1980. The Chinese tallow tree as a cash and petroleum substitute crop. Pp 97-112. IN: Tree crops for energy co-production on farms. U. S. Department of Energy, Solar Energy Research Institute, CP-622-1086. Washington, DC. Scheld, H. W., J. R. Cowles, C. R. Engler, R. Kleiman, & E. B. Schultz, Jr. 1984. Seeds of Chinese tallow tree as a source of chemicals and fuels. Pp 97-111. IN: E. B. Schultz, Jr. and R. P. Morgan [eds.], Fuels and chemicals from oil seeds: Technology and policy options. AAAS Selected symposium 91. Westview Press, Boulder, Colorado. Seibert, M., G. Williams, G. Folger, & T. Milne 1986. Fuel and chemical co-production from tree crops. Biomass 9:49-66. Singh, R. P. & A. Pal 1990. Development and structure of seeds in Chinese tallow tree Sapium sebiferum Roxb. (Euphorbiaceae). Flora 184:15-20. Skinner, M.W. 1998. Personal communication. Cardinals (Cardinalis cardinalis) seen feeding on Chinese tallow (Sapium sebiferum) seeds in Calcasieu Parish, Louisiana, on January 31, 1998. Westbrooks, R. G. & J. W. Preacher 1986. Poisonous plants of eastern North America. University of South Carolina Press, Columbia, South Carolina. 172 p.