Chinese Privet
Scientific Name: Ligustrum sinense Lour.
| General Information | |
|---|---|
| Usda Symbol | LISI |
| Group | Dicot |
| Life Cycle | Perennial |
| Growth Habits | ShrubTree, |
| Native Locations | LISI |
Plant Guide
Uses
Weed (very invasive in the southern US), ornamental
Noxiousness
Chinese privet was introduced into the United States from China for ornamental planting. Having escaped from cultivation, it is now naturalized throughout the southeastern United States. The greatest threat posed by this species is large-scale ecosystem modification due to its ability to successfully compete with and displace native vegetation. Chinese privet plants mature rapidly and are prolific seed producers. They also reproduce vegetatively by means of root suckers. Once established, Chinese privet is difficult to eradicate because of its reproductive capacity. Impact/Vectors: Ligustrum sinense is native to China and was introduced into the United States in 1852 for use as an ornamental shrub. It is used for hedge and mass plantings, and sometimes as single specimens for its foliage and its profusion of small white flowers (Dirr 1990; Wyman 1973). It continues to be widely sold in the nursery and gardening industry. The foliage of Chinese privet is also used, presumably, for cut-flower arrangements. This horticultural introduction has been cultivated for a relatively long time in the United States. Wyman (1973) reports that this species is still growing as a hedge on the old Berkman’s Nursery grounds in Augusta, Georgia, where it was planted in the early 1860’s. It was planted on the Chickamauga and Chattanooga National Military Park after it came under the control of the Secretary of War in 1890. Present day plants are descendants of those early plantings (Faulkner et al. 1989). According to Small (1933), the species was escaping from cultivation in southern Louisiana by the 1930’s. A survey of appropriate herbaria reveals collection records from Georgia as early as 1900. Based on herbarium records the species has become naturalized and widespread in the southeast and eastern U.S. during the 1950’s, 60’s, and 70’s. Taylor et al. (1996) notes the rapid, recent spread of Ligustrum sinense in Oklahoma. © J.S. Peterson USDA, NRCS, NPDC The species is a major threat to natural landscapes. An example of Chinese privet’s ability to push a native species closer to extinction is noted in the recovery plan for Schweintz’s sunflower (Helianthus schweinitzii). This endangered species is known from about 16 populations on the piedmont of the Carolinas. Residential and commercial development and the invasion of aggressive exotics, such as Ligustrum sinense, represent the greatest threats for this species (U.S. Fish and Wildlife Service 1992). Similar observations about the competitive characteristics of Chinese privet have been noted in various Nature Conservancy reports in the Southeast. Removal of Chinese privet from natural areas is problematic and essential for their restoration (News from Volunteers of the Nature Conservancy, North Carolina Chapter and the Louisiana Chapter, pers. comm. 1997). In addition to the privet’s impact on natural landscapes, it can be directly harmful to humans. All introduced species of Ligustrum produce fruit toxic to humans that cause such symptoms as nausea, headache, abdominal pain, vomiting, diarrhea, weakness, and low blood pressure and body temperature. Where Chinese privet occurs in abundance, floral odors may cause respiratory irritation (Westbrooks & Preacher 1986). Chinese privet is sold in nurseries and is often included on recommended planting lists or other literature produced by cooperative extension services without mention of its invasive nature. Named cultivar selections have been developed (Bailey and Bailey 1976). Chinese privet grows in a wide variety of habitats and can tolerate a wide range of soil and light conditions, but it grows best in mesic soils and abundant sunlight but can tolerate lower light conditions (Thomas 1980; Bailey & Bailey 1976). Few woody plants offer an easier test of gardeners’ skills. The species persists on abandoned home sites and can readily invade abandoned lots and farmlands where it forms impenetrable thickets. It becomes especially abundant along fencerows, stream, bayou, and forest margins, and it has the ability to invade forests (Godfrey 1988). Chinese privet reproduces by sexual and vegetative means. Seeds, produced in great abundance, are spread by birds (McRae 1980). Landscape plantings provide seed sources for establishment in disturbed habitats. Soil disturbances of all sorts such as forest clearing, abandoned agricultural lands, and fence construction provide opportunities for colonization by Chinese privet. Natural disturbances for example tree falls, erosion, animal excavations, etc. provide similar colonization opportunities. The plants also have the ability to reproduce vegetatively from root suckers. Once established, Chinese privet is difficult to control because of the huge seedbank and the need to remove underground parts as well. Because of these characteristics, the major impact of Chinese privet is its ability to displace native species and disrupt various terrestrial ecosystems.
Status
Please consult the PLANTS Web site and your State Department of Natural Resources for this plant’s current status, such as, state noxious status and wetland indicator values.
Description
General: Olive Family (Oleaceae). Chinese privet is a shrub or small tree that may grow to as much as 30 feet tall although its typical height ranges from 5 to 12 feet. If flowering, its blossoms are very aromatic. Its root system is shallow but extensive. Suckers are readily produced and the plants can spread vegetatively in this fashion. The plants branch abundantly and the branches typically arch gently downward. Its twigs are usually densely hairy (pubescent) when young, and the plant hairs (trichomes) spread at right angles from the twig surface. Raised, tan-colored lenticels are also evident on the twig’s surface. Chinese privet leaves are evergreen to semi-deciduous and are oppositely arranged (two leaves per node) along the stem on nodes that are usually less than one inch apart. The leaf stalk (or petiole, shown below) is about one- © L. Urbatsch Petiole, axillary bud, stem with spreading © L. Urbatsch Leaves with developing fruit. eighth inch long and covered with hairs. Leaf blades are elliptical in shape and are up to one inch wide and about two inches long. Leaf margins lack teeth (entire). The upper leaf blade surfaces are glabrous (without hairs) at maturity. Hairs occur along the midvein (see photo below) and sometimes on branch veins of the lower surfaces. The flowers occur in numerous, cone-shaped, branching clusters (panicles) two to four inches long that profusely cover the shrub when flowering. A short, slender stalk (pedicel) supports each flower. The green calyx consists of four sepals fused to form a small, cup-like structure. Four white to off-white petals that are basally fused to one another make-up the corolla. Each flower has two stamens attached to the corolla, and they project beyond the corolla throat (exserted stamens). The flowers produce a somewhat disagreeable aroma. The single pistil in each flower matures into a blue-black, berry-like fruit. The fruit are ellipsoidal to nearly globose and are produced abundantly in persistent, pyramidal clusters. Chinese privet is similar to Common Privet (Ligustrum vulgare), a European species that is naturalized in more temperate areas of the eastern United States. Chinese privet has evergreen to semi-evergreen leaves, densely hairy twigs and petioles, pubescent midveins on its lower leaf surfaces, and exserted stamens. In contrast, common privet is deciduous to somewhat evergreen with sparsely pubescent twigs, glabrous midveins, and included stamens (the tips of the anthers are shorter than the extended corolla lobes) (Gleason 1952). Distribution: A survey of herbarium records shows that its present distribution includes an area extending from Florida to southern New England and westward to the eastern parts of Kansas, Oklahoma, and Texas. Chinese privet thrives in wet to dry habitats. It persists around old home sites and flourishes along fencerows, and stream and forest margins where it forms impenetrable, monocultural thickets. For current distribution, please consult the Plant Profile page for this species on the PLANTS Web site.
Control
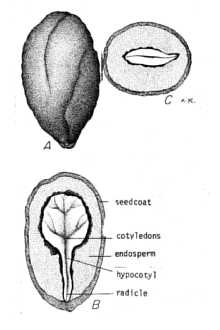
It is recommended that you contact your local agricultural extension specialist and/or county weed specialist for control measures pertinent to your area. © L. Urbatsch Developing fruit & cuplike calyx of fused petals. Various control measures have been reported for Chinese privet. For small areas and for relatively small plants, hand removal is effective. Digging tools such as a mattock are useful for removing underground parts. Broken root fragments need to be removed because of their ability to re-sprout. Repeated mowing and cutting will control the spread of privet, but will not eradicate it. For such treatment, stems should be cut as close to the ground as possible (Bartlow et al. 1997). Mechanical removal is especially effective in the early stages of an invasion when the numbers of plants are relatively small. © L. Urbatsch Midvein of lower leaf surface showing hairs. For larger natural areas where the use of chemical herbicides is inadvisable, enlisting numerous helpers to mechanically remove Chinese privet may be required. Using heavy equipment for large-scale removal may be appropriate in some locations, but the negative effects of soil disturbance and the potential for erosion need to be considered. Herbicide treatments properly applied can selectively remove invasive species with minimal soil disturbance. Even slight soil disturbance may offer opportunities for re-invasion. When considering chemical control, local laws affecting herbicide use must be observed. Appropriate precautions in various habitats may be needed. Kline & Duquesnel (1996) point out that not all herbicides are appropriate for all areas. Some may damage non-target species. Herbicides will behave differently in different environments and under different conditions (Neal et al. 1986). For example, they may degrade more slowly in wetter, more anaerobic soils or move downward in sandier soils. A careful monitoring program is essential for evaluating herbicide use. © L. Urbatsch Petiole, leaf base & margin. Randall & Marinelli (1996) report effective control of Chinese privet with glyphosate herbicides stating that foliage treatment is best for actively growing plants. Foliar spray methods should be used only where risk to non-target species is minimal. A 2% solution of glyphosate or 2% triclopyr with a one-half percent of non-ionic surfactant is reportedly effective for treating Chinese privet (Bartlow et al. 1997). Kline & Duquesnel (1996) discuss various treatments for woody species including Brazilian pepper, Australian pine, Chinese tallow, and other tree-like species. They note that within mixed stands single stem treatments consisting of basal-bark treatments, cut-surface treatments (injection, cut-stump, or girdle), or direct foliar applications may be effective. A typical basal or cut-surface treatment consists of a 10-50% mixture of one of the following types of herbicides (glyphosate, hexazinone, imazapyr, or triclopyr) with an oil dilutant. They provide a table for use as a guide for selecting application methods and herbicides for various invasive plant species. Brian Bowen, President of the Tennessee Exotic Pest Plant Council, reports success in controlling privet using 25% glyphosate/75% horticulture oil applied as a cut-surface treatment (personal communication, 1997). He advises against using this application as the plants break dormancy because upward movement of the sap reduces the treatment’s effectiveness. The same herbicide preparation is effective when applied to cut stumps as long as the ground isn’t frozen (Bartlow et al. 1997). For the basal bark method, applying a mixture of 25% triclopyr/75% horticultural oil to the basal parts of the shrub is reported (Bartlow et al. 1997). W. N. Kline, Senior Scientist, Dow Elanco, Duluth, Georgia, also favors basal-bark or cut-surface treatment over foliar application (pers. comm. 1997). The latter causes such rapid leaf drop that translocation of the herbicide in the plants is reduced, thereby lowering its effectiveness. Furthermore, he reports that disturbance (e.g., fire or mechanical) should be avoided for about one year following basal-bark or cut-surface treatments to allow translocation of herbicides. Disturbance of the plants or root system too soon after treatment may disrupt translocation and result in resprouting. Fire is a naturally occurring phenomenon that is essential for certain native plant communities to exist. Its use in exotic pest plant control is being investigated. Faulkner et al. (1989) reported its effectiveness as a management tool in the Chickamauga and Chattanooga National Military Park for controlling Ligustrum sinense and other pest plants. Fire had the benefit of killing large privet stems, but the vigorous resprouting that followed burning offset this gain. Fall and winter burns had desirable aesthetic effects by considerably reducing the biomass of privet, but no long-term benefits were achieved since the species still remained. Fire was also used as a herbicide pretreatment (Faulkner et al. 1989). In the spring following the fall and winter burns, foliar application of glyphosate damaged or killed a majority of the Chinese privet shoots. Burning facilitated foliar application of herbicide by reducing biomass. However, it did not increase the effectiveness of the herbicide compared to the unburned controls. Privet has no known biological controls. A foliage-feeding insect native to Europe, Macrophya punctumalbum, is a known pest. Privet is also susceptible to a fungal leaf spot, Pseudocercospora ligustri, and a common root crown bacteria, Agrobacterium tume-faciens (Bartlow et al. 1997). Illustrations and Photographs: Auburn University 1999. Dendrology: Chinese privet. Version: 000330. <http://sofserv.forestry.auburn.edu/samuelson/dendrology/oleaceae_pg/chinese_privet.htm>. Department of Forestry, Auburn, Alabama. Gleason, H. A. 1952. Illustrated flora of the northeastern United States and adjacent Canada. Lancaster Press, Inc., Lancaster, PA. (line drawing, vol 3, p. 53.) Godfrey, R. K. 1988. Trees, shrubs, and woody vines of northern Florida and adjacent Georgia, and Alabama. The University of Georgia Press, Athens. 734 pp. (excellent line drawing showing flower and fruit in detail, p. 518). Radford, A. E., H. E. Ahles, & C. R. Bell 1968. Manual of the vascular flora of the Carolinas. University of North Carolina Press, Chapel Hill, North Carolina. (small line drawing, p 831). Randall, J. M. & J. Marinelli 1996. Invasive plants, weeds of the global garden. Brooklyn Botanic Garden, Handbook #149, Brooklyn, New York. 99 p. (photograph, plants in flower, p. 58) Schopmeyer, C. S. 1974. Seeds of woody plants in the United States. USDA, Forest Service, Agricultural Handbook No. 450. (illustration of L. sinense seeds and seedlings of L. vulgare, a similar species, p. 500, 502).
References
Bailey, L. H., & E. Z. Bailey 1976. Hortus third: A concise dictionary of plants cultivated in the United States and Canada. Macmillan Publishing Company, New York, New York. 1186 p. Bartlow, J., K. Johnson, M. Kertis, T. Remaley, S. Ross, E. Simet, T. Smith, D. Soehn, & G. Taylor 1997. Tennessee exotic plant management manual. 119 pp. (http://webriver.com/tn-eppc). Dirr, M. 1990. Manual of woody landscape plants: their identification, ornamental characteristics, culture, propagation, and uses. 4th Edition. Stipes Publishing Co., Champaign, Illinois. 826 pp. Gleason, H. A. 1952. Illustrated flora of the northeastern United States and adjacent Canada. Vol. 3. Lancaster Press, Lancaster, Pennsylvania. Godfrey, R. K. 1988. Trees, shrubs, and woody vines of northern Florida and adjacent Georgia, and Alabama. The University of Georgia Press, Athens, Georgia. 734 pp. James, T. K. & J. Mortimer 1983. Control of privet. Pg 206-209. IN Proceedings of the 37th New Zealand weed and pest control conference, Christ Church, New Zealand. Kline, W. N. & J. G. Duquesnel 1996.
Management
of invasive exotic plants with herbicides in Florida, Down to Earth, Vol 51, No, 2, McRae, W, A, 1980, Unusual bobwhite foods on abandoned Piedmont farmlands, Georgia, Georgia Journal of Science 38(1):49-54, Miller, J,H, 1998, Primary screening of forestry herbicides for control of Chinese privet (Ligustrum sinense), Chinese wisteria (Wisteria sinensis), and trumpetcreeper (Campsis radicans), IN Proceedings, 51st annual Southern Weed Science Society meeting, January 26-28, Birmingham, Alabama, <http://www,srs,fs,fed,us/pubs/viewpub,asp?ID=836>, USDA, FS, Southern Research Station, Ashville, North Carolina, Neal, J, C,, W, A, Skroch, & T, J, Monaco 1985, Effect of plant growth stage on glyphosate absorption and transport in Ligustrum and blue Pacific juniper, Weed Science 34(1):115-121, Randall, J, M, & J, Marinelli 1996, Invasive plants, weeds of the global garden, Brooklyn Botanic Garden, Handbook #149, Brooklyn, New York, 99 p, Westbrooks, R, G, & J, W, Preacher 1986, Poisonous plants of eastern North America, University of South Carolina Press, Columbia, South Carolina, 172 p, Small, J, K, 1933, Manual of the southeastern flora, The University of North Carolina Press, Chapel Hill, North Carolina, 1499 p, Taylor, C, E,, K, Use soil moisture sensors to measure the soil moisture of Chinese Privet., L, Magrath, P, Folley, P, Buck, & S, Carpenter 1996, Oklahoma vascular plants: Additions and distributional comments, Proceedings of the Oklahoma Academy of Science 76:31-34, Thomas, E, H, 1980, The New York Botanical Garden illustrated encyclopedia of horticulture, Garland STPM Press, New York, New York, USDI, Fish and Wildlife Service 1992, Endangered and threatened species of the Southeastern United States (The Red Book), USFWS, Southeastern Region, Atlanta, Georgia, Wyman, W, 1973, Shrubs and vines for American gardens, MacMillan Publishing Co,, Inc,, New York, New York, 613 pp,
