Rush Skeletonweed
Scientific Name: Chondrilla juncea L.

| General Information | |
|---|---|
| Usda Symbol | CHJU |
| Group | Dicot |
| Life Cycle | Perennial |
| Growth Habits | Forb/herb |
| Native Locations | CHJU |
Plant Guide
Alternate Names
skeletonweed, naked weed, gum succory.
Uses
Rush skeletonweed is palatable and nutritious in the rosette and early bolting stages and makes good sheep and goat fodder. Honey bees use it for pollen and honey.
Status
Rush skeletonweed is a non-native, invasive terrestrial forb listed as noxious, prohibited, or banned in nine western states.
Weediness
This plant may be weedy or invasive in some regions or habitats and may displace desirable vegetation if not properly managed. Consult with your local NRCS Field Office, Cooperative Extension Service office, state natural resource, or state agriculture department regarding its status and use. Additional weed information is available from the PLANTS Web site at plants.usda.gov. Consult other related web sites on the Plant Profile for this species for further information.
Description
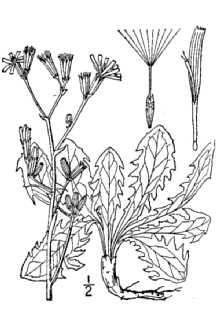
Rush skeletonweed forms a rosette of prostrate, glabrous leaves 1.6 to 24.7 inches (4-12 centimeters) long, 0.6 to 1.8 inches (1.5 to 4.5 centimeters) wide, and oblanceolate in shape. The leaf margins are deeply and irregularly toothed with lobes pointing backward toward the leaf base (runcinate) similar to the rosette leaves of dandelion (Taraxacum spp). The leaf base narrows to a short, winged petiole. Normally, one flowering stem grows per rosette. Flowering stems reach heights of 1.6 to 3.3 feet (50-100 centimeters) and have numerous spreading or ascending branches. They are glabrous except for short, rigid, downward-pointing hairs near the base, similar to prickly lettuce (Lactuca sirriola). Generally, the stems are leafless, they may have long-linear, bract-like leaves, or they may have leaves similar to the rosette leaves but smaller and only on the lower part of the stem. The rosette leaves die at flowering leaving a skeleton-like stem. The flowerheads (capitula) are solitary or in groups of two to five in the stem branch axils, along the branches, and at the branch ends. The cylindrical involucre has two rows of bracts; the outer row is very short and crown-like, the inner row has seven to nine linear-lanceolate bracts with either no hairs, sparsely tomentose, or sometimes a row of rigid hairs on the median line. Each capitulum bears nine to 12 bright yellow, ligulate florets. The florets produce achenes (small fruits) three to four millimeters long and with numerous ribs. At the tip of the achene is a beak five to six millimeters long that bears a pappus of numerous soft bristles. The pappus facilitates wind dispersal. The taproot of rush skeletonweed is small in diameter but penetrates deeply into the soil. Lateral roots are produced along its entire length. Rosettes can grow from adventitious buds at the top of the tap root and along
Distribution
For current distribution, consult the Plant Profile page for this species on the PLANTS Web site.
Habitat
Rush skeletonweed originated from the Transcaspian region of Eurasia, its native range extending from Western Europe and northern Africa to central Asia. It has become widespread in wheat growing regions and rangelands of Idaho, Oregon, and Washington. It is considered an early seral species invading disturbed areas in crop, pasture, range and forest lands. It may also invade intact plant communities in low rainfall areas such as southwestern Idaho. Optimum climatic conditions for rush skeletonweed are cool winters and warm summers without severe drought and with winter and spring precipitation typical of semi-arid and Mediterranean climates. It has been found in areas with annual precipitation ranging from 9 to 59 inches (23 to 150 centimeters) and elevation ranging from sea level to 6,000 feet. Summer temperatures of at least 59 degrees Fahrenheit (15 degrees Celsius) are needed for flower and seed production. Rush skeletonweed has no absolute requirement for vernalization although it accelerates flowering. It is found on a wide range of soil types but is most abundant on sandy, sandy-loam, and silt loam soils. It is a weed of cultivated sites, open areas and disturbances. Areas affected by wildfire and pastures weakened by drought, overgrazing, or with cheatgrass or medusahead invasion are susceptible to rush skeletonweed invasion.
Life History
In its native Eurasian range, rush skeletonweed is described as a biennial. In its invaded ranges in Australia and North and South America it is described as a perennial living up to 20 years. There are also variations in its form. The root system is long lived and rich in carbohydrate reserves. Adventitious buds on the roots enable it to grow year after year. New plants can arise from intact roots or root fragments and local population expansion is mainly by vegetative regeneration. One to several rosettes grows from adventitious root buds of the parent plant usually in autumn (September into November). Plants overwinter as rosettes and begin growth again in the spring (March and April). Rosettes can begin growth in summer if moisture follows drought and rosettes that initiate growth in summer usually flower immediately. Flowering stems elongate from the central growing points of rosettes in April and May. As the flowering stems grow the rosette leaves die leaving nearly leafless plants during the summer. Flower buds form in June and July, and plants bloom in July. The capitula open early in the morning and close before sunset. In hot dry conditions, the capitula will only remain open for a couple of hours. Seeds form without pollination (apomixis). Seeds are fully developed about two weeks after flowering and a small number of seeds have been observed to germinate three days after flowering. Seed production peaks in July and August but can continue into November. Seed production per plant under field conditions can be as high as 10,000, and seed production from dense populations were estimated to be 70,000 per square meter. Flowering stems usually die in October at about the time new rosettes begin to appear; however the timing is variable depending on moisture conditions. Rush skeletonweed flower. Photo by Tim Prather, University of Idaho. Rush skeletonweed seed is not long-lived in the seedbank because they have little to no dormancy and they only remain viable in the soil for 6 to 18 months. However, an Idaho study found that 60% of seed stored for one year maintained good viability. The seed has high viability (up to 80%). Burial of seed deeper than 25 millimeters in the soil prevents germination. Seedlings may emerge at any time moisture is available and temperatures are above 45 degrees Fahrenheit (7 degrees Celsius), but most germination occurs in the fall. As little as 5 millimeters of rain will stimulate germination. Seedlings require a continuous supply of moisture for three to six weeks to survive desiccation. Above-ground growth of seedlings is slow in the fall, but seedling roots grow rapidly. Plants overwinter as rosettes. Rosettes developed from autumn-emerged seedlings usually produce a flowering stem the spring following emergence.
Establishment
Rush skeletonweed establishes from seed and adventitious buds on roots.
Management
See Control.
Pests and Potential Problems
See Environmental Concerns.
Environmental Concerns
Concerns
Concerns
In Australia, rush skeletonweed is considered the most serious weed in wheat growing regions where it reduces yields and the wiry flowering stems, or their latex, clog harvesting equipment which increases breakdown and maintenance costs. Infestations reduce grazing forage potential, the stems interfere with livestock grazing, and there have been reports of the stems causing choking when eaten by cattle. Dense infestations reduce native plant diversity.
Seeds and Plant Production
Plant Production , Use soil moisture sensors to measure the soil moisture of Rush Skeletonweed.
Plant Production
Not applicable.
Control
Contact your local agricultural extension specialist or county weed specialist to learn what works best for control in your area and how to use it safely. Always read label and safety instructions for each control method. Trade names and control measures appear in this document only to provide specific information. USDA NRCS does not guarantee or warranty the products and control methods named, and other products may be equally effective. Rush skeletonweed’s ability to regenerate from roots deep in the soil profile along with poor translocation of herbicide to the extensive root system makes this weed difficult to control with herbicides. Successful control of this plant using herbicides usually requires multiple reapplications. The poor soil conditions favored by the plant (e.g., dry, coarse, and low in organic matter) also reduce herbicide persistence in the soil. Additionally, the morphology of rush skeletonweed, specifically the lack of leaf area, reduces herbicide translocation as a result of inadequate retention and adsorption. Translocation can be improved with silicone surfactants and water conditioning agents. Picloram (one quart product per acre) or picloram combined with 2,4-D (one quart plus one quart per acre) applied to autumn rosettes are the herbicide treatments that give the best root killing results. A single application is not likely to kill all root buds and applications in subsequent years will be necessary. Clopyralid, aminopyralid, and dicamba also translocate into the roots. Hand pulling and digging can provide control of small populations if plants are pulled several times each year for many years. Hand pulling will stimulate adventitious growth from root buds for the first few years until root reserves are depleted. Six to 10 years of mechanical control will be needed to eliminate populations. Mowing is not an effective control for rush skeletonweed. Rosettes are flat to the ground and missed by the mower blade. Mowing when plants bolt to flower may temporarily reduce seed production but plants will survive to flower again. Root fragments of rush skeletonweed are spread by tillage which may increase infestation size. Tillage every six to eight weeks may effectively eliminate the weed. In many locations in Idaho, Montana, Oregon, and Washington, rush skeletonweed does not occupy sites where tillage is practical. Irrigation is not recommended as a control by itself because it stimulates seedling and rosette emergence. Where rush skeletonweed invades irrigated pasture and hayland, carefully planned irrigation management will stimulate the competitiveness of the forage crop and when combined with nutrient, forage harvest, and grazing management practices will help prevent the re-establishment of rush skeletonweed after other control practices are applied. Rush skeletonweed produces larger and leafier rosettes, but not more rosettes, when nitrogen fertilizer is applied. One study found application of superphosphate (about 125 pounds per acre) reduced rosette densities by an average of 80%, probably due to increased competition from pasture plant species. Rush skeletonweed survival relies on a lack of competition, which is of greater importance than increased nutrient levels. That said, nutrient management of hay lands and pastures will stimulate desired plant vigor and reduce the risk of invasion by rush skeletonweed. A study in Idaho shrub-steppe communities found a nearly six-fold increase in rush skeletonweed rosette emergence where wildfires burned compared to non-burned sites the autumn following the burn. Insulated by the soil, rush skeletonweed roots are protected from killing heat of fire. There was also greater seed germination on fire-affected soil compared to unaffected soil. The disturbance of fire produces conditions favorable to rush skeletonweed invasion and population expansion. Prescribed burning should not be conducted in or near areas where rush skeletonweed has invaded unless follow-up management is applied. Rush skeletonweed is good forage for sheep and goats because it is palatable and nutritious in the rosette and early bolting stages. Continuous grazing in the spring and summer will keep it in the rosette stage, but it will quickly flower if grazing is discontinued. Continuous grazing of larger populations is a good strategy to prevent flowering and seed production and thus restrict spread to distant sites along wind currents. Many populations throughout the Intermountain West are small and therefore prescribed grazing as a control may not be practical. However, prescribed grazing is recommended as a preventative management by maintaining a competitive pasture or rangeland plant community. Three biological control agents have been released to manage rush skeletonweed but they have been successful only in certain locations. The skeletonweed root moth, Bradyrrhoa gilveolella, was introduced in Idaho in 2002 but establishment has not been confirmed by 2009. The rush skeletonweed gall midge, Cystiphora schmidti, was first released in California in 1975 and is available for mass collection in California, Idaho, and Oregon. It damages rosettes and flowering stems reducing seed production. The rush skeletonweed rust fungus, Puccinia chondrillina, is the first exotic plant pathogen to succeed as a classical biological control agent in North America by reducing rush skeletonweed to “tolerable levels.” It is readily available for redistribution in California, Idaho, Oregon, and Washington. The effectiveness of biocontrol agents vary depending on local conditions and plant genotype. The rust appears more effective in California and the mite appears to be more important in eastern Washington. Rush skeletonweed is not tolerant of shade and is seldom found on closed forest canopy sites. Disturbance is favorable to rush skeletonweed and removal of natural vegetation provides opportunities for establishment. Revegetation of disturbances is therefore an important measure to provide competition and hinder rush skeletonweed invasion. The use of legumes in crop-pasture rotations has been effective in reducing populations of the weed. The deeply-rooted alfalfa is advantageous because it is competitive for deep soil moisture. Alfalfa also increases soil fertility and plant competition to reduce rush skeletonweed populations. Cultivars, Improved, and Selected Materials (and area of origin) Not applicable.
References
Kinter, C.L., B.A. Mealor, N.L. Shaw, and A.L. Hild. 2007. Postfire invasion potential of rush skeletonweed (Chondrilla juncea). Rangeland Ecology and Management. 60(4): 386-394. Panetta, F.D. and J. Dodd. 1987. The biology of Australian weeds 16. Chondrilla juncea L. The Journal of Australian Institute of Agricultural Science 53(2): 83-95. Piper, G.L., E.M. Coombs, G.P. Markin, and D.B. Joley. 2004. Rush skeletonweed. In: Coombs, E. M., Coombs, J. K. Clark, G. L., and A. F. Cofrancesco, Jr. (eds) Biological control of invasive plants in the United States. Oregon State University Press, Corvallis. 293-303. Sheley, R. L., J.M. Hudak, and R.T. Grubb. 1999. Rush skeletonweed. In: Biology and Management of Noxious Rangeland Weeds. Oregon State Press. Pages 350-361. Prepared By: James S. Jacobs, Plant Materials Specialist Montana/Wyoming, USDA NRCS, Bozeman, MT Kim Goodwin, Weed Prevention Coordinator, Montana State University, Bozeman, MT Daniel G. Ogle, Plant Materials Specialist Idaho/Utah, USDA NRCS, Boise, ID Species Coordinator: James S. Jacobs, Plant Materials Specialist Montana/Wyoming, Bozeman, MT Citation Jacobs, J., Goodwin, K., Ogle, D. 2009 Plant Guide for rush skeletonweed (Chondrilla juncea L. Published October, 2009 ). USDA-Natural Resources Conservation Service, Montana State Office, Bozeman, MT 59715 Edited: 11Aug09JSJ; 12Aug09DGO For more information about this and other plants, please contact your local NRCS field office or