Nutgrass
Scientific Name: Cyperus rotundus L.
| General Information | |
|---|---|
| Usda Symbol | CYRO |
| Group | Monocot |
| Life Cycle | Perennial |
| Growth Habits | Graminoid |
| Native Locations | CYRO |
Plant Guide
Alternate Names
nutgrass, nutsedge, coco sedge, cocograss, red nut sedge, coquito, souchet rond.
Uses
Ethnobotanic: Purple nutsedge has been used in traditional medicine and in landscaping in China. There are reports of its use in India as a soil binder. It is undesirable as fodder, because it quickly becomes fibrous with age, but in the absence of more desirable plants, it can serve that purpose (Holm et al. 1977). Extracts and compounds isolated from purple nutsedge have medicinal properties such as the reduction of fever, inflammation, and pain. The literature contains numerous references to the use of this plant’s roots for essential oils and its seeds for food products. Tuber extracts may reduce nausea and act as a muscle relaxant (Wills 1987). © J.R. Manhart Noxiousness: Purple nutsedge, has been called the “world’s worst” weed. A befitting designation for a species known from more countries (at least 92) than any other weed that infests at least 52 different crops worldwide (Holm et al. 1977). It grows in all types of soils and can survive the highest temperatures known in agriculture. In the United States, purple nutsedge infests cultivated fields, waste areas, roadsides, pastures, and natural areas. It is considered a headache for the southern gardener because of its insidious, rapid growth in flowerbeds and vegetable gardens. Purple nutsedge produces an extensive system of underground tubers from which they can regenerate. Nutsedge is very difficult to control once it is established. Purple nutsedge greatly impacts agriculture and has an unfavorable effect on natural ecosystems by displacing native plants or by changing the availability of food or shelter for native animals. Although relatively small in stature, purple nutsedge provides formidable resource competition for much larger crop plants and ornamentals. This rapid growing plant can quickly form dense colonies due to its ability to produce an extensive system of rhizomes and tubers. Many studies document reduced yields in sugar cane, corn, cotton, rice, vegetables, and numerous other crops. The abundantly produced tubers present an efficient means of dispersal and reproduction. These features together with the ineffectiveness of herbicides make this weed nearly indestructible. Impact/Vectors: Reduction in crop yields is one of the greatest impacts of this species. In extreme cases purple nutsedge can reduce sugarcane yields by 75% and sugar yields by 65%. In Australia, in experimental plots with cultivation, sugarcane yield was reduced by 38%. In Colombian cornfields, when purple nutsedge was allowed to grow for 10 days, yield was reduced by 10%. If allowed to remain for 30 days, yield dropped to 30%. Similar dramatic effects of this weed on cotton, corn, tomatoes, tobacco, mulberries, lemons, and many other crops have been demonstrated (Holm et al. 1977). Rochecouste (1956) noted that even in humid regions the production of purple nutsedge shoots and tubers could severely restrict water availability to sugarcane. Approximate quantities of fertilizer that may be mobilized and stored in purple nutsedge equal 815 kilograms of ammonium sulfate, 320 kilograms of potash, and 200 kilograms of phosphate per hectare (Holm et al. 1977). Besides resource competition, evidence suggests that organic substances released from the decay of dead subterranean tissues may be allelopathic and reduce crop yields where purple nutsedge infestations are severe. Purple nutsedge may produce up to 40,000 kilograms of subterranean plant material per hectare. Under experimental conditions, barley yield was reduced by 15 to 25% by Cyperus rotundus residues in the soil (Horowitz & Friedman 1971). Tuber and rhizome production are important factors in this species’ success as a weed. Rhizomes provide the major means by which the plants may colonize an area. Tubers offer a mechanism for asexual reproduction, and they are the major dispersal unit that can survive extreme conditions. Tubers make the plant difficult to control, because only translocated herbicides are potentially effective on this species. Rhizomes and tubers form extensive networks in the soil. While most tubers are found growing in the upper 15 to 20 cm of soil, a few penetrate to a depth of 40 cm. The root system in heavy clay may extend more than a meter deep (Andrews 1940; Smith and Fick 1937). Under favorable conditions, a single tuber could produce 99 tubers in 90 days (Rao 1968). Experimental plantings of tubers set on 0.9 meter centers resulted in their nearly five-fold increase by the end of the growing season (Hauser 1962). Tubers resist all but high temperature extremes, but seem more sensitive to lower temperatures. Germination failed in tubers held for 12 hours at 500 C. However, greater than 80% germination occurred after exposure to 400 C. Tubers exposed to temperatures of -5 0C or lower did not survive more than two hours (Ueki 1969). Tubers and basal bulbs serve as vegetative propagules. They are carried on farm tillage implements and may be spread by erosion and running water. Severe storms may bring tubers to the surface and transport them to new areas. Such propagules may also be transported long distances with nursery stock. Even though purple nutsedge flowers abundantly, it rarely produces viable seeds. Seeds, although of little reproductive significance in the southern United States, are disseminated by wind or water, transported in mud, or carried onto fields by flooding streams or with irrigation water (Wills 1987; Holm et al. 1977).
Status
Please consult the PLANTS Web site and your State Department of Natural Resources for this plant’s current status, such as, state noxious status, and wetland indicator values.
Description
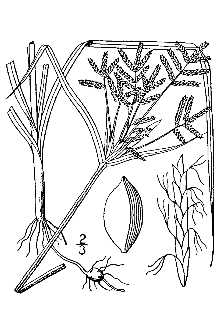
General: Purple nutsedge is a colonial, herbaceous, perennial with fibrous roots that typically grows from 7-40 cm tall and reproduces extensively by rhizomes and tubers, The rhizomes are initially white and fleshy with scaly leaves and then become fibrous, wiry, and very dark brown with age, Rhizomes may grow in any direction in the soil, Those growing upward and reaching the soil surface become enlarged forming a structure 2-25 mm in diameter variously called a “basal bulb, a tuberous bulb, or a corm” that produces shoots, roots, and other rhizomes, Rhizomes that grow downward or horizontally form individual tubers or chains of tubers, Individual tubers are dark reddish-brown when mature, about 12 mm thick, and vary from 10-35 mm long, © Lowell Urbatsch Leaf bases, The dark green, shiny, three-ranked leaf blades arise from or near the base of the plant, They are narrow and grass-like ranging in size from 5-12 mm wide to 50 cm long and have a prominent channel in cross section, The leaf sheaths are tubular and membranous and attach to compact nodes at or near the base of the plant, The upright culms or stems are 10-50 cm tall, smooth, triangular in cross section, and support a much-branched inflorescence, Two to four leaf-like bracts subtend the inflorescence which is umbel-like consisting of 3-9 unequal length branches (sometimes referred to as rays) bearing spikes of 3-10 spikelets, Spikelets are flattened and linear ranging in length from 10-30 mm long, and generally dark reddish purple or reddish brown in color, Each of the 20 or so flowers (florets) in a spikelet are each subtended by a keeled scale (glumes) 2-5 mm long that have a green midvein and a membranous margin, The flowers are bisexual each with three stamens and a pistil bearing three stigmas, Fruit, although rarely produced, consists of a three-angled achene (nutlet), Purple nutsedge possesses the C4 photosynthetic apparatus, which is an adaptation to assimilating CO2 at higher temperatures and higher light intensities compared to C3 pathway plants, C4 plants typically exhibit their best growth rates at temperatures characteristic of tropical and subtropical regions, The leaf anatomy for purple nutsedge is of the Krantz-type, Sheaths of cells that form around the vascular bundles serve to compartmentalize the photosynthetic events, Greater anatomical and physiological details for purple nut sedge are given by Wills (1987), Cyperus esculentus, yellow nut sedge, is another problematic weedy species that reproduces by tubers, It is more widespread and also grows in more temperate parts of the United States, Purple nutsedge is readily distinguished from yellow nut sedge and other sedges by its purplish brown spikelets and scaly or wiry rhizomes that often bear chains of tubers, Distribution: Purple nutsedge is reportedly native to India, but it has been introduced around the World (Holm et al, Use soil moisture sensors to measure the soil moisture of Nutgrass., 1977), The plant is a serious pest in the Southeast ranging from Virginia to central Texas, It also has become established in parts of Arizona and California and has the potential to invade other Pacific states, (Southern Weed Science Society 1995; FICMNEW 1997), This species occasionally occurs in more temperate regions, For example, its presence in Stearns County, Minnesota was documented by a specimen in the University Herbarium collected by J, E, Campbell, July, 1896 (MIN: accession number 81217), but purple nutsedge has not persisted there and other cold locales, The northern limit of nutsedge in Japan is in a region where the average minimum atmospheric temperature is -50C, the temperature below which tubers will not germinate (Ueki, 1969), Temperature appears to limit the species to more tropical and warm temperate regions, For current distribution, please consult the Plant Profile page for this species on the PLANTS Web site, © Lowell Urbatsch Tubers, roots, & leaves,
Control
For control measures that are pertinent to your area, please contact your local agricultural extension specialist or your city/county weed control specialist. Mechanical: Moisture loss is detrimental to tubers. Tuber death ensued after moisture content dropped to 15% or less. Tubers left at the surface of dry soil exposed to full sun desicated beyond recovery after 4 days. Under simulated field conditions, tubers at 5 and 10 cm depths in dry soil that were protected from rain but exposed to sunlight were killed after 8 and 12 days at those respective depths (Holm et al., 1977). Purple nutsedge tubers can be destroyed with repeated summer tillage because of their susceptibility to drying. Infested fields plowed or disked at three-week intervals for the entire growing season reduced tuber number by 80%. At four-week tillage intervals, tuber numbers actually increased. Summer dry fallowing is most effective in light, sandy soils but less so in wetter, heavier soils. Springtooth harrows are an excellent implement for this method. Such tillage methods are often impractical, because the land cannot be used for one or more growing seasons. An exception would be where October to June cropping is feasible (Holm et al. 1977). Where tillage is possible, it can give crops a competitive advantage. The use of precision equipment to cultivate as closely as possible, and hand or mechanical thinning can help to reduce nutsedge competition. Nutsedge is susceptible to shading, which reduces vegetative growth and tuber production. Chemical: Purple nutsedge has proved difficult to control with herbicides. To be effective, the herbicide must be translocated throughout the rhizome and tuber network of the plant. Always follow the manufacture’s recommendations for application and observe all precautions when using herbicides. Also, observe applicable local, state, and federal regulations. A few case histories of herbicide control are cited to indicate progress being made in controlling purple nutsedge. Field experiments over a 10 year period were conducted at the USDA, Southern Weed Science Lab, Stoneville, Mississippi, to determine the effects of tillage and herbicide inputs on purple nutsedge control in cotton. Four tillage cotton production systems (conventional, two levels of reduced-tillage and no-tillage) were evaluated with two herbicide (glyphosate) input levels each. For the most part, seed cotton yields were equivalent for the conventional and reduced tillage production systems regardless of the herbicide input level. Seed cotton yields were less in no-till systems 6 of 10 years regardless of herbicide input levels. It was discovered that the timing of glyphosate application, as a preplant foliar treatment, was extremely important. Purple nutsedge control decreased (i.e., nutsedge populations increased) with glyphosate applications 2 to 4 weeks prior to cotton planting. When glyphosate applications were made at planting in the no-tillage plots, higher levels of purple nutsedge control were realized. Based on predicted costs of herbicide and tillage operations, no-tillage cotton production systems saved monetary inputs and increased net profits 8 of 10 years. Effective and efficient purple nutsedge control in cotton can be obtained in reduced-tillage production systems, but additional research is needed to develop more effective systems of purple nutsedge control in no-tillage cotton production system (Bryson 1996). In cotton and corn, alachlor (no longer permitted in cotton in California due to its damaging effects on the crop plants) is effective against yellow nutsedge, but is less effective on purple nutsedge. DSMA and MSMA provide control of purple nutsedge when applied to cotton as directed-sprays after the cotton plants have two or more leaves. If these herbicides accidentally get on the growing point of cotton plants, they will retard the plant's growth. Because nutsedge is sensitive to competition by shade, early chemical control will allow later shading from the cotton canopy to provide additional control. Smart sprayers that detect and spray only infested areas can save on herbicide costs and introduce less pesticide into the environment (Vargas et al. 1997). Preplant treatment with the soil fumigant, metam-sodium, may provide control if applied during summer fallow i.e., allowing the soil to remain undisturbed for approximately 90 days. (Vargas et al.1997). In four recent studies, a combination of the pesticides Telone® C-17 and Tillam® suppresses weeds including purple nutsedge, nematodes, and certain diseases and achieved yields similar to those obtained by fumigation with methyl bromide for Florida tomatoes intended for fresh markets). Nutsedge is very difficult to control once it is established in turf. Therefore, plant in seedbeds that are free of nutsedge where possible. Small localized infestations of nutsedge may be controlled with metham or repeated applications of glyphosate. Maintaining a closed, competitive turf and avoiding overly wet soil will help control purple nutsedge. Reportedly, Manage® Turf Herbicide does an excellent job on yellow and purple nutsedge in lawns. Biological: Efforts at biological control have explored the usefulness of the moth genus Bactra and searches have been made for potentially effective fungi, viruses, and nematodes. Bactra, a natural insect enemy of sedges in the genera Cyperus, Kyllingia, and Scirpus, has been considered well-suited for biological control of sedge species (Frick & Chandler 1978). Species of Bactra are found in many warmer regions of the world where purple nutsedge is a problem. Their larva feed mainly on sedges. They do not cause appreciable damage to their host plants because of delayed seasonal buildup. Numbers of larvae remain low until early August whereas, large numbers are needed in late May and early June to control nutsedge (Frick & Chandler 1978). Over a six year period, studies were made on the effectiveness of introducing large numbers of adults and larvae early in the growing season to enable insect establishment on nutsedge prior to the time of wide use of insecticides in crops (Frick & Chandler 1978). The studies were conducted on cotton at the USDA, Southern Weed Science Laboratory. Early-season augmentation of Bactra populations is a feasible method for controlling purple nutsedge. Large-scale releases would have to use adults because of the difficulties and time consuming nature of releasing larvae. Releases should continue over a six week period beginning after crop planting. Because of the rapid growth rate of nutsedge, five to ten larvae per shoot are needed. The number of moths to release per unit area would be estimated on the basis of a 50:50 sex ratio and an average of 180 eggs per female (Frick and Chandler 1978). Cyperus rotundus is susceptible to rice grassy stunt tenuivirus, rice tungro bacilliform badnavirus, and rice tungro spherical waikavirus (Brunt et al. 1996). Purple nutsedge is an alternate host for the fungi Fusarium and Puccinia canaliculata. It is also infected by the nematodes Meloidogyne, Rotlylenchus, and Tylenchus. Leaf scorch of purple nutsedge is caused by Ascochyta cyperiphthora (Pomella & Barreto 1997). However, none of these agents causes sufficient destruction to provide sufficient control of this weedy plant (Holm et al. 1977). Illustrations and Photographs Gleason, H. A. 1952. Illustrated flora of the northeastern United States and adjacent Canada. Lancaster Press, Inc., Lancaster, Pennsylvania. (line drawing, vol 1, p. 254.). Holm, L. G., D. L. Plucknett, J. V. Pancho, & J. P. Herberger 1977. The world's worst weeds, distribution and biology. East-West Center, University Press of Hawaii, Honolulu. 609 pp. (excellent line drawings of vegetative and reproductive parts). Radford, A. E., H. E. Ahles, & C. R. Bell 1968. Manual of the vascular flora of the Carolinas. University of North Carolina Press, Chapel Hill, North Carolina. (small line drawing, p 175). Texas A&M University 1999. Vascular plant image gallery. Bioinformatics Working Group. <http://www.csdl.tamu.edu/FLORA/gallery/gallery_query.htm>. College Station, Texas.
References
AboElKheir, A. M., Y. F. ElBanna, & M. A. Khafagy 1992. Morphological and anatomical injuries of glyphosate and surfactants to purple nutsedge (Cyperus rotundus L.). Journal of Agricultural Sciences 17:3542-3554. AbuIrmaileh, B. & L. Jordan 1982. Effect of roundup formulation and components on purple nutsedge (Cyperus rotundus). Dirasat 9:105-119. AbuIrmaileh, B. E. & L. S. Jordan 1978. Some aspects of glyphosate action in purple nutsedge (Cyperus rotundus). Weed Science 26:700-703. Aharonov, B., M. Marmelstein, & Z. Feller 1979. Flordon controls Cyperus rotundus in cotton without damage to the crop. Hassadeh 59:1780-1781. AlAli, F. A., S. R. A. Shamsi, & S. M. Hussain 1978. Sprouting and growth of purple nutsedge, Cyperus rotundus, in relation to pH hydrogen-ion concentration and aeration. Physiol. Plant 44:373-376. Alam, S. M. & A. R. Azmi. 1991. Effect of purple nutsedge (Cyperus rotundus) leaf extract on germination and seedlings growth of wheat (cv. Pavon). Pakistan Journal of Weed Science Research 4:59-61. Almeida, F-S-de "Tiririca", 1972. Cyperus rotundus and Cyperus esculentus control (contribution to its study). Agron-Mocambicana, Apr/June, 6(2):149-155. Anderson, W. P. & G. Hoxworth 1987. Yellow nutsedge control in onions with metolachlor. Research Progress Report. Western Society of Weed Science 131. Anderson, W. P.& M.P. Dunford 1970. Control of purple nutsedge with bensulide. Weed Science 18(3):338-340. Andino, V., E. Hernandez, & V. Garcia 1992. The critical period of competition of weed on Virginia tobacco "Speight G.28" variety. Cultivos Agroindustriales (Cuba) 65-75. Andrews, F. 1940. A study of nut grass (Cyperus rotundus L.) in the cotton soil of Gezira. 1. The maintenance of life in the tuber. Annals of Botany, new series, 4:177-193. Anonymous 1975. Biological weapon against purple nutsedge (Cyperus rotundus), Bactra verutana for control in cotton. Agricultural Research 24:3-5. Anonymous 1975. New herbicide eliminates the worst weed of the world (Cyperus rotundus) in East Africa. Balde Branco (Brazil) 17. Anonymous 1983. Common weeds of sugarcane (Cyperus rotundus and Cyperus esculentus). South African Sugar J. 67:454-455. Arevalo, R.A.; E.A. Cerrizuela, & I. Olea 1977. Control of perennial weeds i.e., Sorghum halepense, Cynodon dactylon and Cyperus rotundus, in Argentine sugarcane plantations by the water flood method. Control de malezas perennes por el metodo de inundacion. Rev-Agron-Noroeste-Argent. Tucuman, Facultad de Agronomia y Zootecnia, Universidad Nacional de Tucuman. 14(1/4):101-109. Arjulis, R. 1990. Allelopathic effects of selected weeds on upland rice. Pemberitaan Penelitian Sukarami (Indonesia) 3-5. Baker, R. S. 1985. Nutsedge control in cotton with Scepter. Proceedings Beltwide Cotton Production Research Conferences 204-205. Baker, F. H., A.D. Worsham, & G.L. Jones 1970. Nutsedge control in corn with butylate. Southern Weed Science Society Proceedings 23:131-142. Baker, R.S. 1976. Herbicides applied as a subsurface layer for nutsedge [Cyperus rotundus] control. 29th Proceedings Southern Weed Science Society. pp 151-156. Barker, J. R., D. J. Herlocker, & S. A. Young 1989. Vegetal dynamics in response to sand dune encroachment within the coastal grasslands of central Somalia. African Journal of Ecology 27:277-282. Bayer, D.E. 1987. Tuber dormancy, germination, apical dominance, and translocation in yellow and purple nutsedge. 39th Proceedings California Weed Conference, Sacramento, California. pp. 90-92. Bendixen, L.E 1975. Cytokinin effects induced in purple nutsedge [Cyperus rotundus] by perfluidone [Herbicides, control]. Weed Science 23(6):445-447. Blanco, H. G., R. A. Arevalo, & S. Chiba 1991. Effects of Cyperus rotundus L. on herbaceous cotton plants. Pesquisa Agropecuaria Brasileira (Brazil) 26:169-176. Botha, P. J. 1971. Red nutgrass and its control. [Cyperus rotundus]. Farming South Africa 47(3):53-55. Bowers, R.N. 1987. The nutsedge problem from a producer's viewpoint. Proceedings Beltwide Cotton Prod. Res. Conf. National Cotton Council and The Cotton Foundation, Memphis, Tennessee. p. 347-349. Brown, S.M. 1990. Weed facts: yellow & purple nutsedge. Bulletin of the Cooperative Extension Service, University of Georgia, Nov (1043) 6 p. Bryson, C.T. 1996. Effect of tillage and herbicide input levels on purple nutsedge control in cotton. TEKTRAN. USDA, Agricultural Research Service, Beltsville, Maryland. <http://www.nal.usda.gov/ttic/tektran/tektran.html>. Burgis, D. S. 1969. Phytotoxicity to purple nutsedge (Cyperus rotundus L.) and soil persistence of some hormone type herbicides. Florida State Horticulture Society Proceedings. 82:143-146. Burr, R.J. & G.F. Warren 1972. An oil carrier for increasing purple nutsedge control. Weed Science 20(4):324-327. Canevari, W. M., L. W. Mitich, & G. B. Kyser 1986. Evaluation of post-emergence herbicides for crop phytotoxicity and control of selected weeds in kidney beans. Research Progress Report. Western Society of Weed Science 147-148. Cardoso, E. M. R. 1984. Effects of herbicides on cassava (Manihot esculenta Crantz) crop. Piracicaba 97. Casquero, P., A. M. d. Ron, & A. Rigueiro 1993. Effectiveness of the sulcotrione for Cyperus rotundus L. and other weeds control in maize. Sociedad Espanola de Malherbologia Lugo. Cauro, O. A., A. Castillo, & E. Duarte 1977. Chemical control of nut grass (Cyperus rotundus L.) using contact and translocation herbicides applied to the foliage. Maracay (Venezuela) 10. Darbyshire, R. L. 1986. Herbicides for conifers: What's new. General Technical Report, Rocky Mountain Forest and Range Experiment Station, Ft. Collins, Colorado. pp. 68-70. Derr, J. F. 1991. Tolerance of woody nursery stock to classic (chlorimuron) and harmony (thiameturon). Journal of Environmental Horticulture 9:9-13. Derr, J. F. 1993. Wildflower tolerance to metolachlor and metolachlor combined with other broadleaf herbicides. HortScience 28:1023-1026. Derr, J. F. & B. L. Appleton 1989. Weed control with landscape fabrics. Journal of Environmental Horticulture 7:129-133. Derr, J.F. & J.W. Wilcut 1993. Control of yellow and purple nutsedges (Cyperus esculentus and C. rotundus) in nursery crops. Weed Technology 7(1):112-117. FICMNEW (Federal Interagency Committee for the Management of Noxious and Exotic Weeds) 1997. Invasive plants - changing the landscape of America. Washington, D.C. Frick, K.E.& J.M. Chandler 1978. Augmenting the moth (Bactra verutana) in field plots for early-season suppression of purple nutsedge (Cyperus rotundus) biological control. Weed Science 26(6):703-710. Frick, K.E., R.D. Williams, P.C. Quimby, Jr. & R.F. Wilson 1979. Comparative biocontrol of purple nutsedge (Cyperus rotundus) and yellow nutsedge (Cyperus esculentus) with Bactra verutana under greenhouse conditions. Weed Science 27(2):178-183. GonzalezIbanez, J. 1983. Control of purple nutsedge (Cyperus rotundus L.) with DPX 4129. Journal of Agriculture of the University of Puerto Rico 67:176-178. Hauser, E. 1962. Establishment of nutsedge from space-planted tubers. Weeds 10:209-212. Hays, S.M. 1991. Ten weeds we could live without. Agricultural research. USDA, Agricultural Research Service. Washington, D.C. June 39(6):4-9. Hicks, R. D., D. A. Addison, J. L. Barrentine, R. B. Cooper, J. A. Keaton,R. K. Mann, K. E. McNeill, & J. F. Nicholson 1984. The use of ethalfluralin for weed control in peanuts. Proceedings Southern Weed Science Society 32-35. Holm, L. G., D. L. Plucknett, J. V. Pancho, & J. P. Herberger 1977. The world's worst weeds, distribution and biology. East-West Center, University Press of Hawaii, Honolulu. 609 pp. Holm, L. G., D. L. Plucknett, J. V. Pancho, & J. P. Herberger 1978. Cyperus rotundus L. Cyperaceae, sedge family. FAO Plant Protection Bulletin 26:73-92. Holt, J.S. 1987. Yellow and purple nutsedge: California distribution, biotypes, and seed production. 39th Proceedings of the California Weed Conference, Sacramento, California. pp. 87-89. Horowitz, M. 1972a. Effects of frequent clipping on three perennial weeds, Cynodon dactylon (L.) Pers., Sorghum halepense (L.) Pers. and Cyperus rotundus L. Exp. Agriculture 8(3):225-234. Horowitz, M. 1972b. Growth, tuber formation and spread of Cyperus rotundus L. from single tubers. Weed Research 12(4):348-363. Horowitz, M. & T. Friedman 1971. Biological activity of subterranean residues of Cynodon dactylon, Sorghum halepense, and Cyperus rotundus. Weed Research 11:88-93. Pomella, A. W. V. & R. W. Barreto 1997. Leaf scorch of purple nutsedge (Cyperus rotundus) caused by Ascochyta cyperiphthora sp. nov. 65: 459-468. Plowman, T. C., A. Leuchtmann, C. Blaney, & K. Clay 1990. Significance of the fungus Balansia cyperi infecting medicinal species of Cyperus (Cyperaceae) from Amazonia. Economic Botany 44:452-462. Rao, J. 1968. Studies on the development of tubers in nutgrass and their starch content at different soil depths. Madras Agriculture Journal 55(1):19-23. Richburg, J. S., J. W. Wilcut, & E. F. Eastin 1993. Weed control and peanut (Arachis hypogaea) response to nicosulfuron and bentazon alone and in mixture. Weed Science 41:615-620. Rincon, D. J. & G.F. Warren 1978. Effect of five thiocarbamate herbicides on purple nutsedge (Cyperus rotundus). Weed Science 26(2):127-131. Rochecouste, E. 1956. Observations on nutgrass (Cyperus rotundus) and its control by chemical methods in Mauritius. Pages 1-11. IN: Procedings of the Ninth Congress of the International Society of Sugar Cane Technologists. Shaffer, G. P., C. E. Sasser, J. G. Gosselink, & M. Rejmanek 1992. Vegetation dynamics in the emerging Atchafalaya Delta, Louisiana, USA. J. Ecol. 80:677-687. Shanmugasundaram, E.R.B., G.K. Mohammed-Akbar, & K. Radha-Shanmugasundaram 1991. Brahmighritham, an Ayurvedic herbal formula for the control of epilepsy. J. Ethno-Pharmacology 33(3):269-276. Small, J. K. 1933. Manual of the southeastern flora. The University of North Carolina Press, Chapel Hill. 1499 p. Smith, E.V. 1942. Nut grass eradication studies. III. The control of nut grass, Cyperus rotundus L., on several soil types by tillage. J. American Society of Agronomy 34(2):151-159. Smith, E.V. & G.L. Fick 1937. Nut grass eradication studies. I. Relation of the life history of nut grass, Cyperus rotundus L., to possible methods of control. J. American Society of Agronomy 29(12):1007-1013. Smith, E.V.& E.L. Mayton 1938. Nut grass eradication studies. II. The eradication of nut grass, Cyperus rotundus L., by certain tillage treatments. J. American Society of Agronomy 30(1):18-21. Southern Weed Science Society 1995. Weeds of the United States. Compact disk for Windows® 3.1 or Windows® 95 or higher. 1508 W. University Avenue, Champaign, Illinois. Standifer, L. 1971. Purple nutsedge: world's worst weed? Louisiana Agriculture 14(3):12-13. Standifer, L.C. 1974. Control of purple nutsedge with 2,4-D, paraquat, and dinose b. Weed Science 22(5):520-522. Stovall, M.E. & K. Clay 1991. Fungitoxic effects of Balansia cyperi. Mycologia 83(3):288-295. Suwunnamek, U. & C. Parker 1975. Control of Cyperus rotundus with glyphosate: the influence of ammonium sulphate and other additives. Weed Research 15(1):13-19. Takematsu, T; M. Konnai, T. Akashiba, & N. Seki 1975. Selective control of cyperaceous weeds with K.223. Weed Science 23(1):15-19. Thullen, R.J. & P.E. Keeley 1979. Seed production and germination in Cyperus esculentus and Cyperus rotundus. Weed Science 27(5):502-505. Ueki, K. 1969. Studies on the control of nutsedge (Cyperus rotundus): On the germination of the tuber. Pp. 355-370. IN: Proceedings of the second Asian-Pacific weed control interchange. University of the Philippines, Los Banos. Vargas, R., B. Fischer, S. Wright, & T. Prather 1997. Integrated pest management guidelines: Cotton. UC DANR Publication 3339. <http://axp.ipm.uc davis.edu/PMG/r114700211.html>. Watson, A. K. 1986. Integrated weed management: moving away from a single strategy. Agrologist 15:21. Webb, J. W., J. D. Dodd, & B. H. Koerth 1981. Plant invasion on upland dredged material. Texas Journal of Science 33:169-183. Westbrooks, R. G. & J. W. Preacher 1986. Poisonous plants of eastern North America. University of South Carolina Press, Columbia, South Carolina. 172 p. Wilcut, J. W. & C. W. Swann 1990. Timing of paraquat applications for weed control in Virginia-type peanuts (Arachis hypogaea). Weed Science 38:558-562. Wilfret, G.J. & D.A. Burgis 1976. Nutsedge (Cyperus rotundus L.) control using herbicides under fallow conditions. Proc. Southern Weed Science Society 29:237-243. William, R.D. & G.F. Warren 1975. Competition between purple nutsedge [Cyperus rotundus] and vegetables. Weed Science 23(4):317-323. Williams, R. D. 1982. Growth and reproduction of Cyperus esculentus L. and Cyperus rotundus L. Weed Res. 22:149-154. Wills, G.D. 1977. Nutsedge [Cyperus rotundus] deals misery to cotton growers. Weeds Today 8(2):16-17. Wills, G.D. 1987. Biology of purple and yellow nutsedge. Proc. Beltwide Cotton Producers Res. Conf., Memphis, Tennessee. National Cotton Council and The Cotton Foundation. p. 352-354. Wills, G. D. 1987. Description of purple and yellow nutsedge (Cyperus rotundus and C. esculentus). Weed Technology 1:2-9. Wilson, C. & T. Whitwell 1993. Tolerance of nineteen species of container grown landscape plants to postemergence applications of Basagran. Journal of Environmental Horticulture 11:86-89. Wilson, H. P. 1978. Control of yellow nutsedge (Cyperus esculentus) with basagran. Vegetable Growers News 32:3-4. Zandstra, B.H; C.K.H. Teo, & R.K. Nishimoto 1974. Response of purple nutsedge to repeated applications of glyphosate. Weed Science 22(3):230-232.
