Needlegrass Rush
Scientific Name: Juncus roemerianus Scheele
| General Information | |
|---|---|
| Usda Symbol | JURO |
| Group | Monocot |
| Life Cycle | Perennial |
| Growth Habits | Graminoid |
| Native Locations | JURO |
Plant Guide
Alternate Names
black grass, Roemers rush, black rush, black needlegrass.
Uses
Dense stands of black needlerush form deep fibrous root systems, which provide very good shoreline protection, filter suspended solids, uptake nutrients, and facilitate substrate oxidation. With its range of salinity tolerances, black needlerush is used in tidal estuary restoration along the Atlantic and Gulf coasts. Seed and vegetative parts of black needlerush are utilized by waterfowl, muskrats, nutria, rice rat, marsh rabbit and non-game birds. More than 60 bird species use black needlerush dominated marsh at least seasonally. Ninety percent of the biomass of marsh plants, such as black needlerush, is not consumed by herbivores. Instead, marsh plant biomass is decomposed to microbial biomass. This microbial biomass is available to primary consumers which initiate food webs leading to commercially important fishes and crustaceans. Black needlerush is commonly planted in constructed wetlands, which are used for the treatment of dilute organic wastes. Researchers evaluated three marsh plant species, black needlerush, common reed (Phragmites australis) and bulltongue arrowhead (Sagittaria lancifolia) for tolerance of soil contaminated with diesel oil. Bulltongue arrowhead exhibited the highest level of tolerance, common reed the least tolerance and black needlerush was intermediate.
Status
Please consult the PLANTS Web site and your State Department of Natural Resources for this plant’s current status (e.g. threatened or endangered species, state noxious status, and wetland indicator values).
Description
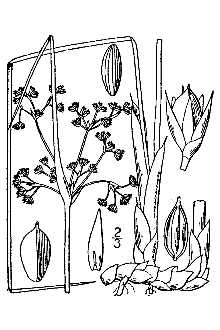
General: Black needlerush is a moderate growing, bunch forming, grass-like perennial. It is course and rigid. Where soil salinity is low, black needle grass is robust with leaves reaching over 2.2 m in height. But in high saline areas the plants are dwarfed often less than 0.3 m tall. Leaves are terete, stiff and pungent. Inflorescence appear laterally; involucral bract terete and erect. Perianth usually brownish, 3-3.5 mm long; sepals longer and are more pointed than petals. It is composed of two types of plants based on flower morphology: one form produces perfect (bisexual flowers) and another form produces pistillate (unisexual) flowers. Seeds are dark and 0.6 mm long (May to October). Black needlerush is found growing in brackish marshes in dense zonal stands. Distribution: Black needlerush is one of the dominate species in the marshes on the southern Atlantic and Gulf coasts. It dominates 20.7% of the marsh in the south Atlantic states and 7.3% of Gulf coast marshes. But, it covers more marsh area on the Gulf coast than on the Atlantic coast. Its distribution is continuous from Maryland to Florida and westward to southwestern Texas. It is usually restricted to coastal
Adaptation
Black needlerush dominated plant communities are classified into three generalized categories based on elevation and salinity influences: 1) Saline marsh, which experiences little dilution of tidal waters; 2) Brackish marsh, where tidal waters are routinely diluted, 3) Intermediate marsh, which is transitional between brackish and freshwater marsh, The number of species in association with black needlerush tends to increase as water salinity decreases, However, Spartina species are thought to be more tolerant of flooding and salinity than black needlerush, In freshwater habitats black needlerush’s growth may be restricted by organisms that feed on its rhizomes, Salt marsh plant communities are characterized by striking zonal patterns across elevation gradients, In a study conduced in Georgia, black needlerush dominated the high elevation marsh and smooth cordgrass (Spartina alterniflora) dominated the middle and low elevation marsh, Black needlerush did not occur naturally in the cordgrass zone and preformed poorly when transplanted there, Use soil moisture sensors to measure the soil moisture of Needlegrass Rush., The poor performance of black needlerush occurred whether or not cordgrass neighbors were removed, which indicates the poor performance was caused by physical stress, In contrast, although smooth cordgrass occurred naturally at low densities in the black needlerush zone, it preformed well there only if the black needlerush neighbors were removed, The excellent performance of smooth cordgrass only where black needlerush neighbors were removed indicates that performance is limited by competition from black needlerush,
Establishment
In the wild, black needlerush establishes from seed, rhizomes or vegetative divisions from storm derived floatsum. Pistillate-flowered plants produce more seed and seed with higher viability than perfect-flowered plants. Seed germination requires light and seeds will not germinate if covered. Seeds remain viable for over a year. Seeds will germinate when submerged or floating on water. Black needlerush seedlings are found mainly in areas with no or little associated vegetation. Seedlings are seldom found in mature stands of black needlerush or other marsh species. Black needlerush maintains itself in established stands though rhizome growth. The percentage germination of seed collected in Mississippi averaged 75 for pistillate flowered plants and 60 for perfect flowered plants. Black needlerush seeds collected from the Mississippi Gulf coast exhibited high percentage germination without a cold-wet stratification. Nursery and greenhouse production are effectively accomplished with seed or vegetative divisions. Seed can be stratified in saturated soil-less potting media with high percentage of fine organics. At the USDA Natural Resources Conservation Service (NRCS) Cape May Plant Materials Center (PMC), New Jersey, seed and media were thoroughly moistened, placed in zip lock bags and stored at approximately 36 ºF for 60 days. Materials were direct seeded into greenhouse starter trays with diurnal temperatures of 80° F days and 50° F nights and 14 hour photoperiods. Seedling emergence was limited and rate of growth slow. For greenhouse forced materials, vegetative divisions use similar environmental photoperiods and diurnal temperatures listed above. Tissue culture and plant regeneration protocols have been developed for blank needlerush.
Management
The effects of fire on blank needlerush dominated marsh vary with water depth and soil moisture. On flooded sites and sites with exposed but saturated soil, fires will not harm the underground reproductive structures. When the mash soil is dry, fire can consume the rhizomes and the entire stand. In Mississippi, burning increased the biomass produced in a black needlerush dominated marsh, but black needlerush recovered more slowly than Spartina species. Three years after burning black needlerush biomass was less than before burning. In Maryland, the biomass and stem densities of several marsh species increased after burning, but burning did not change either the biomass or stem density of black needlerush. In Florida, black needlerush biomass was 47% less one year after burning. In another Florida study, winter burning increased the frequency of common three-square (Schoenoplectus pungens var. pungens) when it occurred as a competing subdominant with black needlerush. But, in nearly pure stands of black needlerush burning did not change the species composition. In general, the effect of burning on black needlerush was either a decrease in biomass and frequency or no change in these parameters.
Pests and Potential Problems
In the mid-Atlantic, several major nursery pests may present themselves and required IPM strategies. These pests can include pythium, brown patch, fungus gnats and algae. This is based upon professional experiences at the NRCS Cape May PMC, New Jersey and diagnosis of pests by Rutgers University Turf Grass Department staff. Consult your local university extension service agent for pesticide recommendations. Cultivars, Improved, and Selected Materials (and area of origin) No improved varieties are known, but numerous wetland nurseries carry local or regional ecotypes. Contact your local Natural Resources
Conservation
Service (formerly Soil Conservation Service) office for more information. Look in the phone book under “United States Government.” The Natural Resources Conservation Service will be listed under the subheading “Department of Agriculture.”
References
Britton, L. and A. Brown, 1970. An illustrated flora of the northern United States and Canada. Dover Press. Brown, M.L. and R.G. Brown. 1984. Herbaceous plants of Maryland. Port City Press. Eleuterius, L.N. 1975. The life history of the salt marsh rush, Juncus roemerianusin. Bull. Torrey Pines Bot. Club. 102: 135-140. Eleuterius, L.N. 1976. The distribution of Juncus roemerianusin the salt marshes of North America. Chesapeake Science 17: 289-292. Eleuterius, L.N.1976. Vegetative morphology and anatomy of the salt marsh rush, Juncus roemerianusin. Gulf Research Reports 5: 1-10. Eleuterius, L.N. 1984. Autecology of the black needlerush Juncus roemerianusin. Gulf Research Reports 7: 27-34. Fell, J.W. and I.L. Hunter. 1979. Fungi associated with the decomposition of the black rush Juncus roemerianus in south Florida. Mycologia 71: 323-342. Fernald, M.L. 1950. Grays manual of botany. 8th Edition. American Book Co. Flores, C. and D. Bonds. Evaluation of vegetative response to fire exclusion and prescribe burning on Blackwater National Wildlife Refuge and
Fishing
Bay Wildlife Management Area. Accessed: 070215. <http://www.fws.gov/blackwater/fire_research.pdf> Gleason, H.A and A.Cronquist. 1963. Manual of vascular plants of the United States and adjacent Canada. Van Norstrand Reinhold Co. Hill, D.T., W.E. Payne, J.W. Jones and S.R. Kown. 1997. Ammonia effects on the biomass production of five constructed wetland plant species. Bioresource Technology 62: 109-133. Hackney, C.T. and A.A. De LaCruz. 1981. Effects of fire on two brackish marsh communities” management implications. Wetlands 1: 75-86. Lin, Q, & I.A. Mendelssohn. 2006. Determination of diesel oil tolerance of coastal dominant marsh plants for restoration and remediation of the oil-impacted habitats. <http://www.osradp.lsu.edu/maySymp06/MaySymp06_Lin.pdf> Meyrs, K.E. 1956. Mangement of needlerush marsh at the Chassahowitzka Refuge. Proc. Annual Conference Southeast Assoc. Game and Fish Comm. 9: 175-177. Odum, W.E., J.C. Zieman and E.J. Heald. 1973. Importance of various plant detritus to estuaries. Pp. 91-114. In: Proceedings of the Costal Marsh and Estuary Management Symposium. Ed. R.H. Chabrech, Louisiana State Univ., Baton Rouge. Pennings, S.C., M. Grant and M.D. Bertness. 2005. Plant zonation in low-latitude salt marshes: disentangling the roles of flooding, salinity and competition. Journal of Ecology 93: 159-167. Stout, J.P. 1984. The ecology of irregularly flooded salt marches of the Northeaster Gulf of Mexico; a community profile. Biol. Rep. 85. U.S. Dept. of Interior, Fish & Wildlife Service. UDSA-Forest Service. Fire effect information system <http://www.fs.fed.us/database/feis/>. Accessed: 070215. Read about Civil Rights at the Natural Resources Conservation Service. USDA, NRCS. 2007. The PLANTS database (http://plants.usda.gov, 31 January 2007). National Plant Data Center, Baton Rouge, LA 70874-4490 USA. Wang, J., D.M. Seliskar and J.L. Gallagher. 2005. Tissue culture and plant rgeneration of the sast marsh monocots Juncus roemerianus and Juncus gerardi. <http://cat.inist.fr/?aModele=afficheN&cpsidt=16953946>.
Fact Sheet
Alternate Names
Black needlerush, black grass, Roemer’s rush.
Uses
Dense stands of black needlerush form deep fibrous root systems, which provide very good shoreline protection, filter suspended solids, uptake nutrients, and facilitate substrate oxidation. With its range of salinity tolerances, black needlerush is used in tidal estuary restoration along the Atlantic and Gulf coasts. Seed and vegetative parts of black needlerush are utilized by waterfowl, muskrats and non-game birds.
Status
Please consult the PLANTS Web site and your State Department of Natural Resources for this plant’s current status (e.g. threatened or endangered species, state noxious status, and wetland indicator values).
Description
Juncus roemerianus is a moderate growing, bunch forming, grass-like perennial. The plant is course and rigid, 0.5-1.5 m tall. The leaves are terete, stiff and pungent. The inflorescence is lateral with an involucral bract that is terete and erect. The perianth is usually brownish, 3-3.5 mm long with sepals longer and is more pointed than petals. Seeds are dark and .0.6 mm long. Black needlerush flowers from May to October; the seed matures from July to November. It is found growing in brackish marshes in dense zonal stands.
Distribution and Adaptation
Adaptation
Adaptation
Black needlerush is one of the dominate species in the marshes on the southern Atlantic and Gulf coasts. It dominates 20.7% of the marsh in the south Atlantic states and 7.3% of Gulf coast marshes. But, it covers more marsh area on the Gulf coast than the Atlantic coast. Its distribution is continuous from Maryland to Florida and westward to southwestern Texas. Black needlerush occupies the edge of ditches and shorelines of bays, back bays and tributaries of tidal systems in coastal systems. It is well adapted to fine and medium textured soils, has a high tolerance to anaerobic conditions, high tolerance to calcium carbonate (CaCO3) and tolerates pH ranges from 4.0-7.0 William B. Skaradek USDA NRCS Cape May Plant Materials Center It is usually restricted to coastal marshes and estuaries, but it may extend 10 to 15 miles inland along river estuaries. For a current distribution map, please consult the Plant Profile page for this species on the PLANTS Website.
Establishment
In the wild, black needlerush establishes from seed, rhizomes or vegetative divisions from storm derived floatsum, Researchers have shown that black needlerush seeds from the Mississippi Gulf coast require light for germination, This seed remains viable for one year and will germinate at a high percentage any time after maturity, and without a cold wet stratification, Nursery and greenhouse production are effectively accomplished with seed or vegetative divisions, Divisions should include several good roots, a node and three to five green stems, Use soil moisture sensors to measure the soil moisture of Needlegrass Rush., Seed can also be stratified in saturated soil-less potting media with high percentage of fine organics, Seed and media can be thoroughly moistened by placing the mix into zip lock bags and stored at approximately 36°F, for 60 days, Materials can be direct seeded into greenhouse starter trays with diurnal temperatures of 80°F days and 50°F nights and 14 hour photoperiods, Seedling emergence was limited and rate of growth slow, For greenhouse forced vegetative divisions use similar environmental photo-periods and diurnal temperatures listed above, Consult your local university extension service agent for pesticide recommendations,
Plant Traits
Growth Requirements
| Temperature, Minimum (°F) | 17 |
|---|---|
| Adapted to Coarse Textured Soils | No |
| Adapted to Fine Textured Soils | Yes |
| Adapted to Medium Textured Soils | Yes |
| Anaerobic Tolerance | High |
| CaCO3 Tolerance | High |
| Cold Stratification Required | No |
| Drought Tolerance | None |
| Fertility Requirement | Medium |
| Fire Tolerance | Medium |
| Frost Free Days, Minimum | 200 |
| Hedge Tolerance | None |
| Moisture Use | High |
| pH, Maximum | 7.0 |
| pH, Minimum | 4.0 |
| Planting Density per Acre, Maxim | 11000 |
| Planting Density per Acre, Minim | 3450 |
| Precipitation, Maximum | 60 |
| Precipitation, Minimum | 40 |
| Root Depth, Minimum (inches) | 14 |
| Salinity Tolerance | High |
| Shade Tolerance | Intolerant |
Morphology/Physiology
| After Harvest Regrowth Rate | Slow |
|---|---|
| Toxicity | None |
| Resprout Ability | No |
| Shape and Orientation | Erect |
| Active Growth Period | Spring |
| Bloat | None |
| Coppice Potential | No |
| Fall Conspicuous | No |
| Fire Resistant | No |
| Flower Color | Green |
| Flower Conspicuous | No |
| Foliage Color | Dark Green |
| Foliage Porosity Summer | Moderate |
| Foliage Porosity Winter | Porous |
| Fruit/Seed Color | Brown |
| Nitrogen Fixation | None |
| Low Growing Grass | No |
| Lifespan | Long |
| Leaf Retention | No |
| Known Allelopath | No |
| Height, Mature (feet) | 4.9 |
| Growth Rate | Moderate |
| Growth Form | Bunch |
| Fruit/Seed Conspicuous | No |
| Foliage Texture | Coarse |
Reproduction
| Vegetative Spread Rate | None |
|---|---|
| Small Grain | No |
| Seedling Vigor | Medium |
| Fruit/Seed Period Begin | Summer |
| Seed Spread Rate | Rapid |
| Propagated by Tubers | No |
| Propagated by Sprigs | Yes |
| Propagated by Sod | No |
| Propagated by Seed | Yes |
| Propagated by Cuttings | No |
| Propagated by Container | No |
| Propagated by Bulb | No |
| Propagated by Bare Root | No |
| Fruit/Seed Persistence | Yes |
| Fruit/Seed Period End | Fall |
| Fruit/Seed Abundance | Medium |
| Commercial Availability | Routinely Available |
| Bloom Period | Spring |
| Propagated by Corm | No |
Suitability/Use
| Veneer Product | No |
|---|---|
| Pulpwood Product | No |
| Post Product | No |
| Palatable Human | No |
| Palatable Graze Animal | Low |
| Nursery Stock Product | No |
| Naval Store Product | No |
| Lumber Product | No |
| Fodder Product | No |
| Christmas Tree Product | No |
| Berry/Nut/Seed Product | No |
